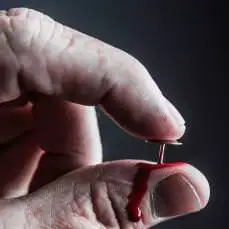
Approximately how many people world-wide suffer from a chronic pain disorder? How common is suicidal ideation in chronic pain victims? ChatGPT-4 on the SCN9A Gene
("the volume knob for pain")
Towards a more civilised signalling system
Answers to prompts by webmaster DP to ChatGPT4 July 2023
"Chronic pain is a significant public health issue affecting millions of people worldwide. According to the World Health Organization, it's estimated that approximately 20% of adults suffer from pain globally, and 10% are newly diagnosed with chronic pain each year. This translates to hundreds of millions of individuals.The prevalence of suicidal ideation in individuals suffering from chronic pain is notably higher than in the general population. A systematic review and meta-analysis published in the Journal of Pain in 2014 found that the prevalence of suicidal ideation in chronic pain patients was about 20%. Other studies suggest that chronic pain sufferers may be two to three times more likely to have suicidal thoughts compared to those without chronic pain.
However, it's important to note that these are general estimates. The actual prevalence can vary depending on numerous factors, such as the specific chronic pain condition, the intensity and duration of the pain, the individual's personal and social resources, mental health status, and access to appropriate medical care.
Chronic pain not only affects the physical aspect of individuals' lives but also significantly impacts their psychological well-being. This underscores the importance of integrated and holistic approaches in managing chronic pain, which includes addressing mental health considerations, such as the risk of suicide. If you or someone you know is dealing with chronic pain and feelings of despair, it's crucial to reach out to healthcare professionals for help."
What is the SCN9A gene, and what role does it play in pain perception?
The SCN9A gene, also known as the Nav1.7 gene, plays a significant role in the human body's perception of pain. This gene provides instructions for making a part of a protein that forms sodium channels in our body. These channels, specifically known as voltage-gated sodium channels, are found in nerve cells and are crucial for transmitting signals, particularly those related to pain sensation. The specific channel produced by the SCN9A gene, called Nav1.7, is found predominantly in peripheral nerves, which are nerves outside the brain and spinal cord, including those that sense pain and temperature.These Nav1.7 channels are particularly important for the initiation and conduction of pain signals. When part of your body is injured, your nerve cells respond by opening these sodium channels. Sodium ions flow into the cell, generating an electrical signal. This signal then travels along the nerves to your brain, which interprets it as pain. Mutations in the SCN9A gene can lead to a variety of conditions that affect pain sensitivity:
Therefore, understanding the role of the SCN9A gene in pain perception could be crucial for developing novel pain management strategies. In fact, drugs that can selectively target and inhibit Nav1.7 channels are being investigated for their potential use as analgesics, or painkillers."
- People with loss-of-function mutations, which result in a non-working Nav1.7 channel, have a condition called congenital insensitivity to pain (CIP). They do not feel pain from any noxious stimuli, including things that would be extremely painful for people without this mutation, such as broken bones or burns.
- On the other hand, gain-of-function mutations, which result in overactive Nav1.7 channels, can lead to conditions characterized by episodes of severe pain, such as erythromelalgia or paroxysmal extreme pain disorder.
Why does The Hedonistic Imperative (hedweb.com) advocate that all prospective parents should be given access to preimplantation genetic screening, counselling and germline editing to ensure pre-selection of benign alleles of the SCN9A gene ("the volume knob for pain") for their future children?
"The Hedonistic Imperative is a philosophical work by David Pearce that promotes the use of biotechnology to abolish suffering in all sentient life. Pearce advocates for the use of genetic engineering and other advanced technologies to eliminate suffering and enhance wellbeing.One of the primary reasons the Hedonistic Imperative advocates for access to preimplantation genetic screening, counseling, and germline editing related to the SCN9A gene (which is sometimes called "the volume knob for pain") is to reduce or eliminate the potential for suffering. Variations in the SCN9A gene can influence how much physical pain an individual experiences. Certain variants can lead to conditions such as congenital insensitivity to pain, where a person cannot feel physical pain, or to the opposite extreme where a person has a much higher sensitivity to pain.
In advocating for benign alleles of the SCN9A gene, the Hedonistic Imperative is essentially advocating for a world where people do not suffer from physical pain, or where they experience it in a more reduced, manageable way. This would, in theory, improve the overall quality of life and wellbeing of individuals.
In terms of genetic counseling and screening, these processes could ensure prospective parents understand the implications of these genetic variations and make informed decisions about the potential genetic makeup of their children.
However, it's important to note that these ideas involve significant ethical, societal, and technical challenges. The potential for misuse of genetic editing technology is a concern, and there are ongoing debates about the morality of altering the human genome in this way. Additionally, our understanding of the human genome and the exact effects of manipulating specific genes like SCN9A is still incomplete."
Describe the evolutionary origin of the scn9a gene.
"The SCN9A gene is part of a larger family of genes known as the voltage-gated sodium channel (Nav) family, which evolved over hundreds of millions of years to carry out critical functions in the nervous systems of animals. These genes encode protein channels that allow sodium ions to pass through cell membranes in response to changes in voltage, which is crucial for the conduction of electrical signals along nerve cells.The Nav gene family is believed to have first appeared in the common ancestor of cnidarians (a group of animals that includes jellyfish and sea anemones) and bilaterians (a group that includes most other animals, including humans) over 500 million years ago. This ancestral gene is thought to have duplicated several times over the course of evolution, resulting in the nine different Nav genes found in humans today, one of which is SCN9A.
SCN9A specifically encodes the Nav1.7 channel, which is primarily expressed in the peripheral nervous system, especially in pain-sensing neurons. Changes and mutations in this gene have been linked to various human pain disorders, including congenital insensitivity to pain and primary erythromelalgia.
Given the role of SCN9A in pain sensation, it likely played a role in the evolution of nociception (the ability to sense harmful or potentially harmful stimuli) and pain perception, which serve crucial functions in helping animals avoid injury and survive. Over evolutionary time, different species may have evolved variations of the SCN9A gene and the Nav1.7 channel that were best suited to their particular environmental challenges and needs.
It's important to note that while we know quite a bit about the broad strokes of the evolution of the Nav gene family, our understanding of the specifics, including the evolutionary history of SCN9A in particular, is still developing. Future research may provide more precise insights into the evolution of this important gene."
How does the SCN9A gene influence sensitivity to pain?
"The SCN9A gene provides instructions for the production of a sodium channel protein, known as Nav1.7. This sodium channel plays a crucial role in the transmission of electrical signals in neurons, particularly in peripheral neurons involved in pain sensation.Variations or mutations in the SCN9A gene can alter the function of the Nav1.7 channel and significantly affect an individual's sensitivity to pain.
Given the critical role of the SCN9A gene and Nav1.7 channels in pain sensation, these areas are key targets for research and drug development efforts aimed at better understanding and managing pain."
- Loss-of-Function Mutations: When there are changes in the SCN9A gene that result in a non-functional or absent Nav1.7 channel, this leads to a condition known as Congenital Insensitivity to Pain (CIP). Individuals with this condition are unable to feel physical pain, even in response to typically painful stimuli such as injury or inflammation. This is because the loss of the Nav1.7 channel disrupts the normal transmission of pain signals from the peripheral nerves to the brain.
- Gain-of-Function Mutations: On the other hand, certain mutations in the SCN9A gene can result in Nav1.7 channels that are overly active or sensitive. These gain-of-function mutations are associated with conditions characterized by extreme pain sensitivity or episodes of intense pain. For instance, conditions such as Erythromelalgia (often characterized by burning pain and redness in the extremities) and Paroxysmal Extreme Pain Disorder (characterized by episodic severe pain in different parts of the body) have been linked to these types of SCN9A mutations.
Can genetic variations in the SCN9A gene lead to altered pain thresholds or pain disorders?
"Yes, genetic variations or mutations in the SCN9A gene, which instructs the formation of the Nav1.7 sodium channel, can lead to altered pain thresholds or pain disorders. These alterations can occur in two primary ways: through loss-of-function mutations and gain-of-function mutations.The influence of SCN9A on pain sensation highlights its potential as a target for new pain relief treatments. If scientists can develop drugs that selectively target the Nav1.7 channel, they might be able to better manage pain in individuals with conditions linked to SCN9A mutations, and potentially in those with other types of chronic pain as well. However, these are still active areas of research."
- Loss-of-Function Mutations: These mutations result in a Nav1.7 channel that is either non-functional or absent. The result is a condition known as Congenital Insensitivity to Pain (CIP). Individuals with CIP are unable to feel physical pain from birth. While this might initially seem beneficial, it can actually lead to a variety of health problems. Without the ability to feel pain, people with CIP may not realize when they are injured, or when a health condition requiring medical attention, such as appendicitis, is developing.
- Gain-of-Function Mutations: These mutations result in a Nav1.7 channel that is hyperactive or overly sensitive, which can lead to conditions characterized by heightened pain sensitivity. An example is Primary Erythromelalgia, a disorder characterized by episodic burning pain and skin redness, usually in the hands and feet. Another is Paroxysmal Extreme Pain Disorder, a condition where individuals experience sudden episodes of severe pain in various body regions.
Which "low pain" alleles of the SCN9A gene are associated with increased pain tolerance but conserved nociception?
"The SCN9A gene is responsible for encoding the alpha subunit of the voltage-gated sodium channel NaV1.7. This channel is found in neurons, including the nociceptive (pain-sensing) neurons, and plays a critical role in the generation and conduction of action potentials. Variations in this gene can affect a person's sensitivity to pain.Most of the research on SCN9A mutations thus far has focused on mutations that lead to extreme pain disorders, such as congenital insensitivity to pain or paroxysmal extreme pain disorder. These disorders are typically associated with either loss-of-function or gain-of-function mutations in the SCN9A gene.
However, there's also research indicating that certain genetic variations of the SCN9A gene are associated with higher pain thresholds in otherwise healthy individuals.
For example, a study by Reimann et al., published in 2010 in the journal "Nature", reported on a particular variant (rs6746030) of the SCN9A gene that results in an amino acid substitution (R1150W) in the Nav1.7 channel. People with the 'W' variant of the SNP (single nucleotide polymorphism) were found to have a higher pain threshold, yet they were not insensitive to pain.
To date, this is one of the best-known examples of a "low pain" variant of the SCN9A gene that is associated with increased pain tolerance but conserved nociception. It's important to note that our understanding of these genetic variations and their impacts on pain perception is still evolving, and future research may uncover more such variants and clarify the mechanisms through which they influence pain perception."
Describe the effects of the rs6746030 variant of the SCN9A gene that results in an amino acid substitution (R1150W) in the Nav1.7 channel. What would be the outcome of ensuring all children had the benign rs6746030 variant as urged by hedweb.com?
"The rs6746030 variant of the SCN9A gene results in an amino acid substitution (R1150W) in the Nav1.7 sodium channel, which is largely expressed in the pain-sensing neurons (nociceptors). This variant appears to enhance the functionality of these channels, making them shut off more rapidly and reducing the propensity for the neuron to fire, therefore making the pain threshold higher than in individuals without this variant. In other words, people with this variant tend to be less sensitive to pain.Still, these individuals are not entirely insensitive to pain, as pain sensation (nociception) is preserved. This suggests that this variant might not eliminate the capacity for pain (which could be harmful, as pain serves a protective function), but rather make individuals more resistant to pain.
The idea put forth by the Hedonistic Imperative (hedweb.com) suggests that if all children had this variant, it could lead to a reduction in suffering because these individuals would be more resistant to pain. This could have wide-ranging impacts, potentially reducing the personal and societal burdens of pain and suffering.
However, there are a number of important caveats to consider:
The technology to perform this kind of widespread genetic editing safely and effectively does not yet exist. While CRISPR and other gene editing tools have made significant strides, there are still substantial technical challenges to overcome, particularly in terms of precision and prevention of off-target effects."
- This is a simplification of a very complex biological system. Pain perception is influenced by multiple genes, not just SCN9A, as well as environmental factors. Altering one gene may not have the desired effect or could have unintended consequences.
- While the reduction of pain might seem beneficial, pain does serve a protective function. It alerts us to potential damage to our bodies, allowing us to respond and avoid further harm. It is unclear what the broader implications might be of widespread reduction of pain sensitivity.
- The ethical implications of widespread genetic editing, especially in humans, are substantial. Such actions raise issues about consent, equity, and potential long-term impacts on human evolution.
What are the potential mechanisms by which mutations in SCN9A can affect pain signaling?
The SCN9A gene provides instructions for producing a protein that is part of a sodium channel known as Nav1.7. Sodium channels, including Nav1.7, play a crucial role in the ability of neurons (nerve cells) to generate and transmit electrical signals. In the case of Nav1.7, it is predominantly located in peripheral neurons - those involved in sensations such as pain, pressure, and temperature.When part of your body is injured, cells in that area release chemicals that are detected by peripheral neurons. In response, the Nav1.7 sodium channels in those neurons open, allowing sodium ions to flood into the cell. This creates a flow of electrical charge, or action potential, that is then transmitted along the nerve to the spinal cord and brain, where it is interpreted as pain.
Mutations in the SCN9A gene can affect the functioning of Nav1.7 channels in different ways:
In this way, mutations in the SCN9A gene can significantly impact pain signaling by affecting the function of Nav1.7 channels. Understanding these mechanisms can aid in the development of new treatments for managing pain, such as drugs that target Nav1.7 channels.
- Loss-of-Function Mutations: These mutations cause the Nav1.7 channel to be non-functional or absent. This prevents the generation of action potentials in response to injury, which disrupts the transmission of pain signals to the brain. As a result, individuals with these mutations have a condition known as congenital insensitivity to pain (CIP), where they cannot feel any physical pain.
- Gain-of-Function Mutations: These mutations cause the Nav1.7 channel to be overly active. This leads to the generation of action potentials even in response to minor stimuli, or spontaneously without any stimulus. The result is an oversensitivity to pain or periods of intense pain. Conditions associated with these mutations include erythromelalgia (characterized by episodes of severe burning pain and redness in the extremities) and paroxysmal extreme pain disorder (characterized by sudden, severe, episodic facial and body pain).
Are there any known therapeutic implications of studying the SCN9A gene in pain management?
Yes, there are several therapeutic implications of studying the SCN9A gene, which provides instructions for making the Nav1.7 sodium channel, a crucial component in the perception of pain. Because mutations in SCN9A can cause pain disorders or insensitivity to pain, the Nav1.7 channel has become a significant focus for pain management research and drug development. The aim is to develop medications that can selectively target Nav1.7 channels, thereby controlling their function and moderating pain perception. Here are a few potential avenues:These potential therapies underscore the potential benefits of studying the SCN9A gene in pain management. However, most of these approaches are still in the research or development phase, and more work is needed to determine their safety and efficacy."
- Drugs that inhibit Nav1.7 channels: The idea here is to use drugs to selectively "block" Nav1.7 channels, thus reducing the perception of pain. Some pharmaceutical companies and researchers are investigating this approach, with several compounds being tested in preclinical and clinical trials.
- Gene Therapy: Another possible approach could be gene therapy to modify the SCN9A gene. This could be potentially beneficial for conditions resulting from gain-of-function mutations in SCN9A, but this approach is largely theoretical and faces significant technical and ethical hurdles.
- Utilizing Animal Venoms: Some animal venoms are known to contain compounds that can block or modulate the function of sodium channels, including Nav1.7. These compounds are being studied for their potential use as painkillers.
- Understanding Opioid Effectiveness: It has been found that in some individuals with loss-of-function mutations in SCN9A, opioids do not have the typical analgesic effect. Studying this interaction can lead to an understanding of why some people do not respond to opioids and help develop more effective treatments.
How does the SCN9A gene interact with other genes or genetic pathways related to pain perception?
The SCN9A gene, which provides instructions for the production of the Nav1.7 sodium channel, plays a vital role in pain perception. Nav1.7 is responsible for the initiation and propagation of action potentials in peripheral sensory neurons, which are responsible for transmitting pain signals.Pain perception is a complex process and involves the interplay of several genes and pathways. While Nav1.7 plays a critical role in the initiation of the pain signal, its transmission, modulation, and perception involve other elements such as other sodium channels, potassium channels, and neurotransmitters.
Here are some ways in which the SCN9A gene can interact with other genes or genetic pathways related to pain perception:
Understanding these interactions is complex and is still an area of active research. However, it can provide a more comprehensive view of pain perception and open up new avenues for the development of analgesics and pain management strategies."
- Interactions with Other Sodium Channels: There are other sodium channel genes, such as SCN10A and SCN11A, which encode for the Nav1.8 and Nav1.9 channels, respectively. These channels are also present in sensory neurons and are involved in the transmission and modulation of pain signals. Changes in these genes can also affect pain sensitivity.
- Potassium Channels: Potassium channels, such as those encoded by the KCNQ and KCNA genes, help to repolarize the cell after an action potential and thus contribute to the regulation of neuronal excitability. Changes in these channels can affect pain signaling.
- Neurotransmitters and Their Receptors: Neurotransmitters such as Substance P, Glutamate, and GABA, along with their respective receptors, play a role in transmitting and modulating the pain signal within the central nervous system. Genes encoding for these neurotransmitters and their receptors can interact with SCN9A in the overall perception of pain.
- Endogenous Opioid System: The body produces endogenous opioids that act on opioid receptors to reduce the perception of pain. The genes coding for these opioids and their receptors can interact with the SCN9A pathway.
Outline recent research advances in understanding the SCN9A gene's involvement in chronic pain conditions
Several advancements have been made in understanding the role of the SCN9A gene in chronic pain conditions. The SCN9A gene codes for the Nav1.7 sodium channel, which plays a crucial role in pain sensation. Both loss-of-function and gain-of-function mutations in SCN9A are known to affect pain sensitivity.Can changes in the SCN9A gene expression or function result in both hypoalgesia (decreased pain sensation) and hyperalgesia (increased pain-sensitivity)?
- Characterization of Pain Disorder: Research has identified various pain disorders linked to mutations in SCN9A, such as primary erythromelalgia and paroxysmal extreme pain disorder (associated with gain-of-function mutations) and congenital insensitivity to pain (associated with loss-of-function mutations). This research helps in better characterization of these disorders, which can lead to more effective diagnosis and treatment strategies.
- Drug Development: Given the vital role of SCN9A in pain sensation, it has become a major target for pain management drugs. Several pharmaceutical companies and researchers are developing and testing drugs that can selectively target the Nav1.7 channel. These compounds aim to modulate the function of Nav1.7 and control pain perception effectively.
- Gene Therapy: Some preliminary research is exploring the potential for gene therapy to modify the SCN9A gene and treat pain conditions. However, this area of research is still in its early stages and faces considerable technical and ethical challenges.
- Animal Studies: A number of animal studies have been conducted to better understand the role of SCN9A in pain sensation. These studies have helped to elucidate the basic biology of Nav1.7 and pain transmission.
- Study of Interactions: Research has also been conducted into how SCN9A interacts with other genes and genetic pathways related to pain perception. This research helps provide a more comprehensive understanding of pain sensation and the role of Nav1.7.
Yes, changes in the SCN9A gene, which encodes the Nav1.7 sodium channel, can indeed lead to both hypoalgesia (decreased pain sensation) and hyperalgesia (increased pain sensitivity). The specific outcome depends on the type of mutation in the gene.It's also worth noting that understanding the specific effects of SCN9A mutations not only improves our understanding of these rare conditions but could also lead to advancements in the development of more effective pain management strategies for more common types of chronic pain. This remains a significant focus of ongoing research.
- Hypoalgesia: Loss-of-function mutations in the SCN9A gene can result in hypoalgesia, or a decreased sensitivity to pain. These are typically nonsense or missense mutations that result in a non-functional Nav1.7 sodium channel. A complete inability to sense pain is a condition known as Congenital Insensitivity to Pain (CIP). Individuals with this condition are born with an inability to feel pain, which can lead to unintentional self-injury, unnoticed infections, and other serious health problems.
- Hyperalgesia: On the other hand, gain-of-function mutations in the SCN9A gene can lead to hyperalgesia, or an increased sensitivity to pain. These mutations result in an overly active or sensitive Nav1.7 sodium channel, which can amplify the transmission of pain signals. Examples of conditions that can result from such mutations include Primary Erythermalgia (PE) and Paroxysmal Extreme Pain Disorder (PEPD), both of which involve episodes of severe, burning pain often triggered by mild stimuli such as warmth or eating.
Are there any specific populations or ethnic groups that exhibit distinct variations or mutations in the SCN9A gene associated with pain-sensitivity?
The SCN9A gene encodes the Nav1.7 sodium channel, which plays a critical role in pain sensation. Both loss-of-function and gain-of-function mutations in this gene can significantly affect pain sensitivity. However, these mutations are typically rare and not limited to or more prevalent in specific populations or ethnic groups.Conditions resulting from these mutations, such as congenital insensitivity to pain (associated with loss-of-function mutations) and primary erythromelalgia or paroxysmal extreme pain disorder (associated with gain-of-function mutations), have been identified in people from diverse ethnic backgrounds.
While there have been studies investigating population-specific or ethnic differences in pain sensitivity, these differences are likely to result from a complex interplay of many genes and environmental factors, rather than variations in a single gene like SCN9A.
However, it's worth noting that genetic studies have historically been skewed towards populations of European descent, and there may be undiscovered genetic variants in underrepresented populations. Thus, while no specific populations or ethnic groups are known to exhibit distinct variations or mutations in the SCN9A gene, future research may uncover new findings in this area."
How do environmental factors interact with genetic variations in SCN9A to influence an individual's pain experience?
The SCN9A gene encodes for a voltage-gated sodium channel known as Nav1.7, which plays a crucial role in the transmission of pain signals from peripheral nerves to the central nervous system. Mutations in this gene can cause various pain disorders, such as primary erythromelalgia, paroxysmal extreme pain disorder, and congenital insensitivity to pain. Environmental factors can interact with these genetic variations to influence an individual's pain experience in several ways:In summary, the relationship between environmental factors and genetic variations in SCN9A is complex and multifaceted. They together influence an individual's pain experience, and understanding this interplay can guide personalized pain management strategies."
- Epigenetic modifications: These are chemical changes to DNA or the proteins associated with it that don't change the sequence itself but influence gene expression. Environmental factors such as diet, stress, smoking, or exposure to toxins can cause these modifications. For example, stress or trauma might lead to changes in DNA methylation or histone modification of the SCN9A gene, thereby affecting its expression and the individual's sensitivity to pain.
- Pain triggers: Environmental factors can also act as triggers for pain in individuals with SCN9A mutations. For example, individuals with primary erythromelalgia (caused by SCN9A mutations) often experience pain attacks triggered by warm temperatures or physical exertion.
- Medication response: The environment, in the form of medication use, can also interact with SCN9A variations. Some people with certain SCN9A mutations may respond poorly to typical analgesics or need higher doses for effective pain relief.
- Lifestyle factors: Regular physical activity, a balanced diet, and good sleep hygiene can generally help to manage chronic pain conditions. Conversely, a sedentary lifestyle, poor diet, or inadequate sleep might exacerbate pain.
- Psychological factors: Chronic pain is not only a physical condition but is also strongly influenced by psychological factors. Stress, anxiety, and depression can intensify the perception of pain. Behavioral therapies and stress management techniques can help improve these conditions, potentially reducing pain.
What are the potential implications of manipulating the SCN9A gene or its related pathways for developing novel pain treatments?
"The SCN9A gene encodes a voltage-gated sodium channel, Nav1.7, which is critical in the transmission of pain signals from peripheral nerves to the central nervous system. The discovery of its role in pain perception has been a major breakthrough in pain research, suggesting that SCN9A and the Nav1.7 channel could be potential targets for new pain treatments. Here are some implications of manipulating the SCN9A gene or its related pathways:While the potential benefits of targeting SCN9A and its related pathways are promising, it's important to keep in mind that more research is needed to fully understand the effects of manipulating this gene, as well as the potential side effects and ethical considerations."
- Development of Novel Painkillers: Researchers are investigating drugs that can specifically block the Nav1.7 channel without affecting other sodium channels. This specificity could potentially reduce the side effects associated with current pain medications, such as opioids, which act on multiple targets and can lead to dependence and tolerance.
- Gene Therapy: Scientists are exploring the use of gene therapy to modify the SCN9A gene in people with mutations that cause extreme pain or insensitivity to pain. For example, for those with a gain-of-function mutation that causes extreme pain, introducing a normal copy of the gene or suppressing the mutated gene could potentially alleviate their symptoms.
- Personalized Medicine: Understanding a person's unique SCN9A gene sequence could allow for personalized pain treatments. Some mutations may respond better to specific types of treatment than others, so genotyping patients could help guide therapeutic decisions.
- Preemptive Analgesia: If a genetic screening suggests that a patient has a high risk of severe postoperative pain due to SCN9A variations, doctors could use preemptive analgesia (pain control methods used before surgery) to manage the pain more effectively.
- Understanding Pain Mechanisms: Studying the SCN9A gene and the Nav1.7 channel can help scientists understand the fundamental mechanisms of pain. This knowledge can pave the way for developing new strategies for pain management.
- Pharmacogenomics: The study of how genes affect a person's response to drugs (pharmacogenomics) could be utilized to predict which patients might be resistant to certain analgesics due to their SCN9A gene variations.
How is the SCN9A gene linked to inherited pain disorders such as erythromelalgia or congenital insensitivity to pain?
The SCN9A gene encodes a voltage-gated sodium channel known as Nav1.7, which is primarily expressed in peripheral sensory neurons involved in pain signaling. Genetic variations in the SCN9A gene can lead to alterations in the function of this channel, resulting in several inherited pain disorders, including primary erythromelalgia and congenital insensitivity to pain.Both conditions highlight the critical role of the Nav1.7 channel, encoded by the SCN9A gene, in pain perception. By understanding how alterations to this gene result in these disorders, researchers hope to develop more effective pain treatments."
- Primary Erythromelalgia: This condition, also known as inherited erythromelalgia, is characterized by episodes of pain, redness, and swelling in parts of the body, especially the hands and feet. This disorder is caused by gain-of-function mutations in the SCN9A gene, which enhance the activity of the Nav1.7 channel. These mutations make the neurons more excitable and lower the threshold for firing a pain signal, leading to the abnormal pain perception seen in individuals with this disorder.
- Congenital Insensitivity to Pain (CIP): This rare condition involves the inability to sense physical pain from birth. It is caused by loss-of-function mutations in the SCN9A gene, which lead to nonfunctional Nav1.7 channels. As a result, pain signals aren't properly transmitted to the brain, rendering these individuals insensitive to pain.
What role does SCN9A play in acute versus chronic pain conditions?
The SCN9A gene encodes the voltage-gated sodium channel, Nav1.7, which plays a crucial role in transmitting pain signals from the peripheral nervous system to the central nervous system. While our understanding of its specific contributions to various types of pain (acute versus chronic) is still developing, some general insights can be made.In conclusion, while SCN9A and its product, Nav1.7, clearly play a crucial role in the perception of pain, further research is needed to fully elucidate their roles in different types of pain and how these can be targeted to develop more effective pain treatments."
- Acute Pain: This is a type of pain that lasts for a short duration, typically resulting from an injury, surgery, or illness. In the context of acute pain, the Nav1.7 channel encoded by SCN9A helps transmit the pain signals in response to the damaging stimuli. When the injury heals, the pain generally resolves.
- Chronic Pain: This type of pain lasts for longer periods, often beyond the normal time for healing. It can result from an ongoing condition (like arthritis) or develop after nerve damage (neuropathic pain). Research has suggested that SCN9A mutations may contribute to certain chronic pain conditions, as they can alter the excitability of pain-sensing neurons and hence change pain perception.
For example, gain-of-function mutations in SCN9A, which increase the activity of the Nav1.7 channel, have been linked to chronic pain disorders such as inherited erythromelalgia and paroxysmal extreme pain disorder. On the other hand, loss-of-function mutations, which reduce or eliminate Nav1.7 activity, cause congenital insensitivity to pain, where individuals cannot feel pain at all.
Moreover, some research suggests that Nav1.7 may play a role in "pain plasticity," where the nervous system undergoes changes in response to injury or disease that can result in chronic pain. For instance, changes in the expression or function of Nav1.7 may occur in response to nerve damage or inflammation, potentially contributing to chronic pain.
Are there any ethical considerations associated with studying or manipulating the SCN9A gene in the context of pain management?
Yes, there are ethical considerations associated with studying and manipulating the SCN9A gene in the context of pain management. Some of these considerations include:As our understanding and ability to manipulate genes like SCN9A grow, it's crucial to engage in ongoing ethical discussions to guide the responsible use of these powerful technologies."
- Genetic Privacy and Confidentiality: As with any genetic research or intervention, there's the issue of privacy and confidentiality of genetic information. Misuse of this information could lead to genetic discrimination in areas such as insurance and employment.
- Informed Consent: Individuals must be thoroughly informed about the purpose, potential risks, benefits, and alternatives of the study or intervention, including potential future uses of their genetic information.
- Equity of Access: Novel treatments resulting from SCN9A research might initially be expensive and thus only accessible to a privileged few. This can exacerbate existing health inequities.
- Unintended Consequences: While the goal of manipulating SCN9A would be to reduce pain, it's important to consider potential unforeseen side effects. For instance, complete insensitivity to pain, while it may seem beneficial, can actually be dangerous, as pain serves a protective function.
- Germline Gene Editing: If the manipulation of SCN9A involves germline gene editing (altering the genes in sperm, eggs, or early embryos), this raises significant ethical issues. Changes made would be inheritable, potentially affecting future generations. The long-term effects of such changes are uncertain.
- Enhancement versus Treatment: Manipulation of the SCN9A gene could potentially be used not only to treat pain conditions but also to enhance human abilities (for example, to create individuals who can withstand extreme conditions). This use of gene editing opens up a range of ethical debates. Animal Research: Much of the research on SCN9A and pain involves animal models. This raises issues of animal welfare and the ethical justification of causing pain or altering pain perception in animals for research purposes.
How do different types of SCN9A gene mutations result in varying pain phenotypes and clinical manifestations?
The SCN9A gene encodes for a voltage-gated sodium channel called Nav1.7, which is found in nerve cells that transmit pain signals. The Nav1.7 channel plays a critical role in generating and propagating action potentials, which are the electrical signals that nerve cells use to communicate.Different types of mutations in the SCN9A gene can change the way the Nav1.7 channel functions, resulting in varying pain phenotypes and clinical manifestations.
Gain-of-function mutations enhance the activity of the Nav1.7 channel, making neurons more likely to fire. This increased excitability can lead to heightened pain sensitivity. Conditions caused by gain-of-function mutations in SCN9A include:
On the other hand, loss-of-function mutations reduce or eliminate the activity of the Nav1.7 channel, making neurons less likely to fire. This can lead to decreased pain sensitivity or even insensitivity to pain. A condition caused by loss-of-function mutations in SCN9A includes:
- Primary Erythromelalgia (PE):This condition is characterized by episodic burning pain, redness, and warmth in the extremities, usually triggered by warmth or exertion. Gain-of-function mutations in SCN9A make the Nav1.7 channel overly responsive, leading to increased neuronal excitability and pain sensation.
- Paroxysmal Extreme Pain Disorder (PEPD): Formerly known as familial rectal pain, PEPD is characterized by bouts of severe pain in various parts of the body, including the rectum, ocular region, and jaw. This is also caused by gain-of-function mutations, although the precise mechanisms leading to the specific symptoms of PEPD may differ from those in PE.
In sum, different types of SCN9A gene mutations can change the function of the Nav1.7 channel in different ways, leading to a spectrum of pain sensitivity disorders. This offers an insight into the mechanisms of pain perception and has implications for the development of novel pain treatments."
- Congenital Insensitivity to Pain (CIP): This rare condition involves complete inability to feel pain from birth. The loss-of-function mutations result in nonfunctional Nav1.7 channels, so pain signals aren't properly transmitted to the brain. People with CIP can have severe injuries or illnesses without realizing it because they don't feel any pain.
What animal models have been used to study the SCN9A gene and its impact on pain perception?
Several animal models, notably mice and rats, have been widely used to study the SCN9A gene and its impact on pain perception.Each of these models has advantages and disadvantages, and the choice of model often depends on the specific research question. These animal models provide crucial insights into the functioning of the SCN9A gene and its role in pain perception, informing the development of new pain treatments."
- Mice Models: Transgenic mice, where a particular gene has been inserted, removed, or altered, are often used in pain research. For example:
- Knockout Mice:Mice have been genetically engineered to "knock out" the Scn9a gene (the mouse equivalent of the human SCN9A gene), rendering them unable to produce the Nav1.7 channel. These mice show a profound reduction in pain responses, modeling conditions such as congenital insensitivity to pain.
- Transgenic Mice: In other experiments, mice have been engineered to carry human SCN9A gene mutations associated with inherited erythromelalgia or paroxysmal extreme pain disorder. These mice show increased pain responses, modeling these conditions.
- Rat Models: Rats have also been used in pain research, particularly in the study of neuropathic and inflammatory pain. Like mice, they can be genetically engineered to model human conditions, and their larger size can sometimes make them more convenient for certain types of experiments.
- Zebrafish Models: While mammals are often preferred for pain research due to their physiological similarities to humans, zebrafish are also used as a model organism to study the function of Nav1.7 and other ion channels. Their transparent bodies during early development stages allow for easy observation of internal processes.
- Drosophila (Fruit Fly) Models: Fruit flies have also been used to study pain signaling, including the role of Nav1.7. Despite the vast evolutionary distance between humans and fruit flies, many of the basic mechanisms of nerve function are surprisingly similar.
Are there any ongoing clinical trials investigating therapies targeting the SCN9A gene for pain relief?
There are indeed clinical trials investigating therapies that target the SCN9A gene and its product, the Nav1.7 sodium channel, for pain relief. A few of these include:While promising, these trials have encountered challenges because blocking Nav1.7 has not always produced the profound analgesia observed in individuals with congenital insensitivity to pain due to SCN9A loss-of-function mutations. This discrepancy may be due to compensatory changes in the nervous system or the need for more selective or complete inhibition of Nav1.7. As new findings continue to emerge, it's always a good idea to check the latest updates on clinical trial databases like ClinicalTrials.gov to get the most recent information on ongoing trials and their progress. Furthermore, gene therapies targeting SCN9A for pain management might also be in the experimental stages, given the rapid advancements in the field of genetics."
- BIIB074 (Vixotrigine): This is a Nav1.7 sodium channel blocker being investigated for use in conditions such as trigeminal neuralgia and small fiber neuropathy. It's thought to work by inhibiting the activity of the Nav1.7 sodium channel, thereby reducing the transmission of pain signals.
- PF-05089771: Pfizer has conducted clinical trials on this selective Nav1.7 inhibitor for pain conditions.
Can genetic testing of the SCN9A gene be used for personalised pain management strategies?
Genetic testing of the SCN9A gene, which encodes the Nav1.7 sodium channel critical for pain signal transmission, could indeed contribute to personalized pain management strategies in the future. There are several potential ways in which this might happen:However, while the potential of personalized pain management based on SCN9A genetic testing is promising, we're still in the early stages of this research. There are many challenges to overcome, including the need for more research to understand the complex relationships between genetic variants, pain perception, and response to treatment. Also, ethical considerations, such as genetic privacy and access to care, must be addressed. However, with continued research and development, SCN9A genetic testing may become an important tool in personalized pain management in the future."
- Identifying Genetic Variants Linked to Pain Sensitivity: Genetic testing can reveal variants in SCN9A that may make a person more or less sensitive to pain. For instance, gain-of-function mutations in SCN9A are associated with conditions characterized by severe pain, such as primary erythromelalgia and paroxysmal extreme pain disorder. Conversely, loss-of-function mutations lead to conditions like congenital insensitivity to pain. Identifying these variants could help predict a person's susceptibility to chronic pain conditions or their likely response to injury or surgery.
- Targeted Therapies: If treatments are developed that specifically target the Nav1.7 channel or the downstream effects of SCN9A mutations, genetic testing could help identify patients who would most benefit from these treatments. For example, if drugs are developed that can specifically block the hyperactive Nav1.7 channels caused by certain SCN9A mutations, these could potentially be used to treat individuals with conditions like primary erythromelalgia.
- Pharmacogenomics: Genetic variants can affect how individuals respond to medications, including pain relievers. While the role of SCN9A variants in response to current pain medications is not yet fully understood, it is possible that this information could be used in the future to guide personalized treatment plans.
How does the SCN9A gene influence the processing of other sensory stimuli besides pain, such as touch or temperature?
The SCN9A gene encodes the Nav1.7 voltage-gated sodium channel, which is predominantly expressed in peripheral sensory and sympathetic neurons. While the gene is most well-known for its role in pain perception, it's also important for the sensation of other stimuli, such as touch and temperature.In the case of Congenital Insensitivity to Pain (CIP), which is caused by loss-of-function mutations in SCN9A, affected individuals can't feel pain but can still feel other sensations like touch and temperature. This indicates that while Nav1.7 is crucial for pain sensation, it's not the only channel involved in the processing of these other sensory stimuli. Other sodium channels and sensory mechanisms also contribute to these complex processes. In summary, while SCN9A's primary role is in pain perception, it also contributes to the processing of other sensory stimuli, including temperature. Its role in touch sensation is less clear and would benefit from further research."
- Temperature: The Nav1.7 channel has a role in sensing changes in temperature. Certain mutations in SCN9A can lead to primary erythromelalgia, a condition where individuals experience severe burning pain often triggered by warmth. This suggests that Nav1.7 has a role in temperature sensation and, when mutated, can lower the threshold for heat-induced pain. Another disorder, called Paroxysmal Extreme Pain Disorder (PEPD), which is also caused by SCN9A mutations, involves episodes of pain often triggered by changes in temperature, further underlining the gene's role in temperature sensing.
- Touch: The role of Nav1.7 in the perception of touch is less understood, but it's known that Nav1.7 is present in the dorsal root ganglia, which contains sensory neurons responsible for transmitting information about touch from the peripheral nervous system to the brain. This suggests a potential role for Nav1.7 in mechanosensation, or the sense of touch. However, more research is needed to elucidate this role.
What are the potential implications of SCN9A gene research for our understanding of the neurobiology of pain?
Research into the SCN9A gene has provided and continues to provide significant insights into our understanding of the neurobiology of pain. Here are a few potential implications:As we continue to learn more about SCN9A and other genes involved in pain perception, we can expect these findings to inform the development of new treatments and strategies for managing pain, helping to improve the lives of individuals suffering from chronic pain conditions."
- Understanding Pain Mechanisms: The SCN9A gene codes for the Nav1.7 sodium channel, which plays a crucial role in the transmission of pain signals. By studying mutations in SCN9A that either heighten or lessen pain sensitivity, we're learning more about how pain signals are generated and transmitted, and how these processes can go awry.
- Identifying New Drug Targets: The Nav1.7 channel is an attractive target for new pain medications. Drugs that can modulate the activity of this channel could potentially provide relief for various pain conditions, especially those currently difficult to treat with existing medications.
- Unraveling Pain Heterogeneity: Pain is a highly individual experience, with different people reporting different levels of pain in response to the same stimulus. Research on SCN9A and other pain-related genes could help explain this variability and lead to more personalized pain treatments.
- Linking Pain and Other Conditions: Some studies suggest that SCN9A mutations may be associated with other conditions such as irritable bowel syndrome, hinting at a broader role for this gene in the sensory nervous system.
- Informing Gene Therapy Approaches: If certain mutations in SCN9A can cause insensitivity to pain without major side effects, it raises the possibility of using gene therapy to induce similar changes to treat severe chronic pain. However, this approach would need to be carefully controlled to prevent complete insensitivity to pain, which can be dangerous.
- Reconsidering Pain Management Strategies: The existence of individuals with SCN9A mutations causing congenital insensitivity to pain, who lead relatively normal lives, suggests that eliminating certain types of pain might be more feasible than previously thought. This could influence the development of novel pain management strategies.
Are there any known pharmacological interventions or drugs that target the SCN9A gene or its related pathways to modulate pain perception?
Several pharmacological interventions are in development or under investigation that target the Nav1.7 channel, encoded by the SCN9A gene, to modulate pain perception. These interventions aim to reduce or block the activity of Nav1.7, thereby reducing the transmission of pain signals. Here are a few examples:While the development of Nav1.7 inhibitors has been challenging, these and other potential treatments represent a promising direction for non-opioid pain management. If successful, they could provide much-needed relief for people suffering from chronic pain conditions.
- BIIB074 (Vixotrigine): This is a non-opioid painkiller developed by Biogen that selectively targets the Nav1.7 channel. In clinical trials, it showed promise for treating conditions like trigeminal neuralgia and small fiber neuropathy.
- PF-05089771: Pfizer developed this selective Nav1.7 inhibitor, which was tested in clinical trials as a potential treatment for pain.
- Funapide (TV-45070): This is a topical Nav1.7 inhibitor developed by Teva Pharmaceuticals and Xenon Pharmaceuticals. In trials, it was being tested for conditions like post-herpetic neuralgia.
- Raxatrigine (GDC-0276) and GDC-0310: These are two oral Nav1.7 blockers developed by Genentech that have undergone clinical trials.
- Toxins: Certain toxins, such as those produced by cone snails (e.g., μ-conotoxins like MrVIB), have been found to selectively inhibit the Nav1.7 channel. While these are not yet used clinically, they provide insight into possible ways to modulate Nav1.7 activity.
These treatments are in various stages of development and testing, and their effectiveness and safety profiles were not fully established."
What are the evolutionary implications of the SCN9A gene and its role in pain perception across different species?
The SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel, is evolutionarily conserved across many species, highlighting its critical role in the functioning of nervous systems. As it pertains to pain perception, here are some evolutionary implications:In summary, the SCN9A gene's conservation and its critical role in pain perception across different species highlight pain's evolutionary importance. Understanding the gene's function and evolution can help us better comprehend pain mechanisms and develop novel pain treatments."
- Conservation of Function: SCN9A and its role in pain perception seem to be preserved across many species. For example, studies have shown that loss-of-function mutations in the mouse version of this gene (Scn9a) result in a lack of pain sensation, similar to the congenital insensitivity to pain seen in humans with similar mutations. This conservation indicates a fundamental role for Nav1.7 in transmitting pain signals across diverse species.
- Adaptive Significance of Pain: Pain perception is an essential survival tool, allowing organisms to respond to harmful stimuli and avoid injury. The widespread presence and conservation of the SCN9A gene and its function in pain perception underline the importance of pain in evolutionary terms.
- Variations and Adaptations: There may be species-specific adaptations in SCN9A and its role in pain perception. For example, some animals seem to have much higher pain tolerance than humans, which could potentially be linked to differences in SCN9A or other pain-related genes. Understanding these differences could provide valuable insights into pain mechanisms and potential therapeutic targets.
- Evolution-Informed Drug Development: Understanding the evolution of SCN9A could inform drug development. For instance, researchers have studied toxins from venomous animals like spiders and cone snails, which have evolved to target sodium channels like Nav1.7, as potential pain therapeutics. By investigating how these toxins interact with Nav1.7, scientists can gain insights into developing drugs that selectively target this channel.
How do epigenetic modifications influence the expression and function of the SCN9A gene in pain modulation?
Epigenetic modifications, which are changes to gene expression without changes to the underlying DNA sequence, can significantly influence the expression and function of genes, including SCN9A. While specific research on SCN9A and epigenetic modifications is still emerging, some general possibilities could be suggested based on what we know about epigenetics and pain:Research on epigenetic modifications of SCN9A and their influence on pain modulation is still developing, and more studies would be needed to clarify these relationships. Understanding how epigenetic changes affect SCN9A could potentially open up new avenues for pain management, such as drugs that modify these epigenetic changes."
- DNA Methylation: Methylation of the DNA, particularly at CpG sites, typically reduces gene expression. If methylation occurs in the regulatory regions of SCN9A, it could potentially reduce the expression of Nav1.7, thus impacting the transmission of pain signals. For instance, if reduced expression of Nav1.7 results in less efficient transmission of pain signals, increased methylation of SCN9A could theoretically result in lower pain sensitivity.
- Histone Modification: Histones are proteins that DNA wraps around, and their modification can affect gene expression. Acetylation of histones generally promotes gene expression, while methylation can either inhibit or promote expression, depending on the context. Changes to the histone code in the SCN9A gene could potentially affect its expression and thus influence pain perception.
- Non-coding RNAs: MicroRNAs and other non-coding RNAs can also regulate gene expression. If certain non-coding RNAs target SCN9A, they could modulate its expression, again potentially influencing pain signaling.
- Epigenetic Changes and Chronic Pain: Chronic pain conditions have been associated with global and gene-specific epigenetic changes. If such changes affect SCN9A, they could potentially contribute to the development or maintenance of chronic pain.
Can changes in the SCN9A gene expression contribute to the development of chronic pain conditions?
Changes in the SCN9A gene expression can potentially contribute to the development of chronic pain conditions, given that this gene encodes the Nav1.7 voltage-gated sodium channel, a critical component in the transmission of pain signals.Chronic pain often arises from altered neuronal activity in the peripheral or central nervous system. As the Nav1.7 channel is involved in generating and propagating action potentials in pain-sensing neurons (nociceptors), alterations in SCN9A expression could affect the excitability of these neurons, potentially contributing to the development of chronic pain. Increased expression of SCN9A could theoretically result in hyperexcitability of nociceptors, leading to heightened pain sensitivity or even pain in the absence of any injury or disease (a condition known as neuropathic pain). Conversely, decreased expression might theoretically result in hyposensitivity to pain.
While this provides a potential link between SCN9A expression and chronic pain, it's important to note that the actual relationship is likely to be much more complex. Pain perception and the development of chronic pain conditions are influenced by many factors, including other genes, environmental factors, and the interplay between different components of the nervous system.
Furthermore, research has shown that specific mutations in SCN9A can lead to rare pain disorders, such as primary erythromelalgia (characterized by episodes of severe pain, usually in the extremities, often triggered by heat or exercise) and congenital insensitivity to pain (an inability to feel pain). These conditions indicate that not just the level of SCN9A expression, but also the specific function of the Nav1.7 channel, plays a key role in pain perception.
More research is needed to fully understand how changes in SCN9A expression contribute to chronic pain conditions. Future studies in this area could potentially lead to new strategies for preventing or treating chronic pain."
What are the potential genetic and non-genetic factors that interact with the SCN9A gene to determine an individual's pain sensitivity?
Pain sensitivity is a complex trait influenced by both genetic and non-genetic factors. With respect to the SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel, various interactions can influence an individual's pain sensitivity:Genetic Factors:
Non-genetic Factors:
- Mutations in SCN9A: Specific mutations in SCN9A can lead to disorders like primary erythromelalgia (characterized by burning pain and redness in the extremities) and congenital insensitivity to pain (an inability to feel pain).
- Polymorphisms in SCN9A: Certain variations in SCN9A that do not necessarily cause disease may nonetheless affect pain sensitivity. For instance, single nucleotide polymorphisms (SNPs) might alter the function of Nav1.7 or its expression level, potentially affecting pain sensitivity.
- Interactions with Other Genes: Pain perception is a complex process involving many genes. Interactions between SCN9A and other pain-related genes could influence pain sensitivity. For example, other genes encoding different types of sodium channels (such as SCN10A/Nav1.8 and SCN11A/Nav1.9) or genes involved in the downstream processing of pain signals could interact with SCN9A to influence overall pain sensitivity.
Understanding the interplay between genetic and non-genetic factors in determining an individual's pain sensitivity can be complex and multifaceted, and it remains an active area of research. It's crucial to note that while SCN9A plays a significant role in pain perception, it's just one piece of the broader picture."
- Environmental Factors: Environmental exposures or stressors could potentially affect SCN9A expression or function. For example, inflammation could potentially alter the activity of Nav1.7 and thus pain sensitivity.
- Epigenetic Modifications: Changes in DNA methylation, histone modification, or non-coding RNA expression could influence SCN9A expression, thereby potentially affecting pain sensitivity.
- Age, Sex, and Hormonal Factors: Age, sex, and hormonal status can influence pain perception. For example, estrogen has been shown to affect the expression of certain sodium channels, and there might be similar effects on SCN9A.
- Psychosocial Factors: Factors such as stress, anxiety, depression, and expectation can significantly influence the perception of pain, potentially interacting with the biological pathways involving SCN9A.
How do genetic variations in the SCN9A gene affect response to pain medications or analgesics?
The SCN9A gene encodes the Nav1.7 voltage-gated sodium channel, a crucial component involved in the transmission of pain signals. Variations in this gene can influence an individual's sensitivity to pain and potentially the response to pain medications.It's important to note that while SCN9A can influence the response to pain medications, it's just one of many factors involved. Other genes, as well as non-genetic factors like age, sex, overall health status, and the type and severity of pain, also play significant roles. Research is still ongoing to fully understand how genetic variations in SCN9A and other genes affect the response to pain medications. This is a promising area of research that could significantly improve pain management in the future."
- SCN9A Mutations and Pain Insensitivity: Certain mutations in the SCN9A gene can result in conditions such as congenital insensitivity to pain, where individuals are unable to feel physical pain. In such cases, traditional pain medications or analgesics may not have any noticeable effects, as these individuals don't experience pain in the same way most people do.
- SCN9A Mutations and Heightened Pain Sensitivity: Conversely, other mutations can lead to conditions like erythromelalgia or paroxysmal extreme pain disorder, where individuals have heightened pain sensitivity. These people might require higher doses of analgesics to achieve the same level of pain relief as individuals without these mutations, or they might find that traditional painkillers are less effective.
- Polymorphisms and Pain Medication Response: Variations in SCN9A that don't cause disease could still influence pain sensitivity and response to medications. For example, certain single nucleotide polymorphisms (SNPs) might alter the function of the Nav1.7 channel or its expression level, affecting how an individual responds to analgesics.
- Potential for Personalized Medicine: Understanding the genetic variations in SCN9A and their effects on pain perception could lead to more personalized pain management strategies. For instance, if certain variations are found to be associated with a better response to specific drugs, genetic testing could be used to guide treatment decisions.
Are there any known neurological or neurodevelopmental disorders associated with SCN9A gene mutations that also involve altered pain perception?
Yes, there are several known neurological disorders associated with mutations in the SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel, and these disorders often involve altered pain perception. They include:It's important to note that these conditions are rare, and most variations in the SCN9A gene do not cause such extreme alterations in pain perception. More research is being done to fully understand the role of SCN9A and other genes in pain perception and to develop potential new treatments for pain based on this knowledge."
- Primary Erythromelalgia (PE): This is a rare disorder that typically begins in childhood and involves episodes of burning pain, redness, warmth, and swelling in the extremities. The pain episodes can be triggered by warm temperatures or mild physical activity. This disorder is associated with gain-of-function mutations in the SCN9A gene, which result in hyperactivity of the Nav1.7 channel.
- Paroxysmal Extreme Pain Disorder (PEPD): Formerly known as familial rectal pain, this disorder is characterized by severe episodic pain in various parts of the body, including the rectal, ocular, and submandibular areas. It is also associated with gain-of-function mutations in the SCN9A gene.
- Congenital Insensitivity to Pain (CIP): This is a condition characterized by an inability to feel physical pain from birth. People with this disorder can have serious injuries, such as broken bones or burns, without realizing it. CIP is associated with loss-of-function mutations in the SCN9A gene, which result in a lack of functional Nav1.7 channels. While these disorders are characterized primarily by altered pain perception, they might also involve other neurological symptoms. For example, people with PEPD can also experience other symptoms, such as flushing, seizures, and abnormalities in heart rate or blood pressure.
What are the implications of studying the SCN9A gene for understanding and treating neuropathic pain conditions?
Neuropathic pain, caused by damage or disease affecting the somatosensory nervous system, is often challenging to treat. Research on the SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel, holds significant implications for understanding and treating neuropathic pain conditions for several reasons:Research on SCN9A and neuropathic pain is ongoing. While this line of investigation is promising, it's important to remember that pain perception is a complex process involving many genes, environmental factors, and interactions between different components of the nervous system. Therefore, SCN9A is just one piece of a much larger puzzle."
- Understanding Pain Pathways: Studying the SCN9A gene and its protein product Nav1.7 helps in understanding the fundamental biological mechanisms of pain transmission. Nav1.7 is a critical component of pain-sensing neurons (nociceptors) and plays a significant role in generating and propagating action potentials, the electrical signals transmitted along nerves.
- Insights into Pain Disorders: Mutations in SCN9A can lead to pain disorders, such as primary erythromelalgia (characterized by episodes of severe pain and redness in the extremities) and congenital insensitivity to pain (an inability to feel pain). Studying these conditions provides valuable insights into the role of SCN9A and Nav1.7 in pain perception, potentially offering clues about how to treat neuropathic pain.
- Potential for New Therapies: If specific SCN9A mutations can eliminate the sensation of pain without severe side effects (as in congenital insensitivity to pain), this suggests that Nav1.7 could be a viable target for new pain medications. Drugs that inhibit Nav1.7 could potentially relieve neuropathic pain without the risk of addiction associated with opioid painkillers.
- Personalized Medicine: Studying SCN9A could also help pave the way for more personalized approaches to pain management. If certain genetic variations are associated with a greater risk of neuropathic pain or a better response to specific treatments, genetic testing could be used to guide therapeutic decisions.
How does the SCN9A gene influence the perception and response to different types of pain, such as inflammatory pain, neuropathic pain, or nociceptive pain?
The SCN9A gene encodes the Nav1.7 voltage-gated sodium channel, a critical component in the transmission of pain signals from the peripheral nervous system to the central nervous system. This sodium channel plays a crucial role in the excitability of peripheral nociceptive (pain-sensing) neurons, impacting the perception and response to different types of pain, including inflammatory pain, neuropathic pain, and nociceptive pain.It's important to note that while SCN9A and Nav1.7 play significant roles in pain perception, they are just one part of a complex network of genes and proteins involved in this process. Other factors, including other genes, environmental influences, and the interplay between different components of the nervous system, also contribute to the perception and response to different types of pain.
- Inflammatory Pain: Inflammatory pain occurs in response to tissue injury and inflammation. The inflammatory process leads to the release of various chemicals that can sensitize nociceptors. Nav1.7 channels, along with other sodium channels, contribute to the generation and propagation of action potentials in these sensitized nociceptors, allowing the transmission of pain signals. Thus, variations in SCN9A that affect the function or expression of Nav1.7 could potentially influence the perception of inflammatory pain.
- Neuropathic Pain: Neuropathic pain arises due to damage to or dysfunction of the nervous system. Nav1.7 channels are important for the normal function of nociceptors, and alterations in these channels can result in abnormal neuronal excitability, potentially contributing to neuropathic pain. For example, certain mutations in SCN9A are associated with disorders like primary erythromelalgia and paroxysmal extreme pain disorder, both of which involve neuropathic pain.
- Nociceptive Pain: Nociceptive pain is the pain perceived in response to noxious stimuli like extreme temperatures, mechanical force, or certain chemicals. Nav1.7 channels help to regulate the response of nociceptors to these stimuli. Loss-of-function mutations in SCN9A can result in congenital insensitivity to pain, a condition in which individuals are unable to perceive nociceptive pain.
Ongoing research is further exploring the role of SCN9A and Nav1.7 in pain perception and their potential as targets for new pain treatments."
Can the study of the SCN9A gene provide insights into the mechanisms underlying placebo and nocebo effects in pain perception?
The placebo effect refers to a perceived improvement in symptoms, such as pain, following administration of a sham or inactive treatment, while the nocebo effect refers to a perceived worsening of symptoms following a similar treatment. These phenomena are believed to be driven largely by psychological factors, including patient expectations, conditioning, and the therapeutic context.While the SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel, plays a critical role in the transmission of pain signals, its direct involvement in the mechanisms underlying placebo and nocebo effects is less clear. The placebo and nocebo effects are complex processes involving various components of the nervous system, including cognitive (e.g., prefrontal cortex), emotional (e.g., amygdala), and reward (e.g., ventral striatum) pathways, as well as neurotransmitter systems like endogenous opioids and cannabinoids.
That said, genetic factors, including genes like SCN9A, could potentially influence the placebo and nocebo responses in pain perception. For instance, if certain SCN9A variations were found to affect the sensitivity to pain, these could also impact the magnitude of placebo or nocebo responses in pain conditions.
More broadly, understanding the genetic influences on pain perception, such as those mediated by SCN9A, could contribute to a more nuanced view of placebo and nocebo effects. For instance, genetic information could potentially be used to predict individual differences in the susceptibility to these effects or to tailor pain management strategies more effectively.
Much of the research into the genetic basis of placebo and nocebo responses is still preliminary, and SCN9A has not been a primary focus of this research. Nonetheless, this is a fascinating area of study that could yield important insights into the biological basis of these phenomena and their role in pain management.
What are the potential challenges or limitations in targeting the SCN9A gene for pain management in a clinical setting?
While the SCN9A gene and its encoded Nav1.7 sodium channel represent promising targets for pain management, there are several potential challenges or limitations in translating this research into clinical practice:Research into SCN9A as a target for pain management is ongoing, and several drug candidates are in development or clinical trials. These efforts represent a promising direction for the field of pain management, but they are likely just the beginning of what will be a long and complex journey to bring these treatments to patients."
- Selective Targeting: The Nav1.7 channel is one of several different sodium channels in the body, some of which perform critical functions in other tissues, including the heart and muscles. Therefore, developing drugs that selectively target Nav1.7 without affecting other sodium channels can be challenging. Non-selective blockade could potentially result in serious side effects.
- Variable Responses: Genetic variations in SCN9A and other genes could influence how individuals respond to treatments targeting Nav1.7. For example, certain individuals might have mutations that make their Nav1.7 channels resistant to these treatments. Understanding these genetic influences and tailoring treatments to individual genetic profiles is a complex process.
- Complexity of Pain: Pain perception is a complex process involving many different genes, environmental factors, and interactions within the nervous system. While SCN9A plays a significant role in pain transmission, it is only one piece of the puzzle. Therefore, even highly effective treatments targeting SCN9A might not completely alleviate pain in all individuals or for all types of pain.
- Drug Development and Approval: The process of developing new drugs and getting them approved for use in humans is long, costly, and fraught with challenges. Even after a potential drug candidate is identified, it needs to undergo rigorous testing in preclinical and clinical trials to determine its safety and efficacy. Many promising drug candidates fail at some stage of this process.
- Ethical Considerations:The prospect of manipulating genes for pain management raises several ethical considerations. These include questions about the potential for misuse, the accessibility of these treatments, and the potential for unintended consequences, such as the elimination of useful pain signals that serve as warnings of injury or disease.
How do lifestyle factors, such as diet or exercise, interact with genetic variations in the SCN9A gene to influence pain sensitivity?
The specifics of how lifestyle factors like diet or exercise interact with genetic variations in the SCN9A gene to influence pain sensitivity aren't well understood. Pain perception is a complex trait influenced by an interplay of genetic, environmental, and lifestyle factors, and the interactions among these are intricate and still being researched. That being said, here are a few general principles that might apply:Despite these potential interactions, it's important to note that SCN9A is only one of many genes that influence pain sensitivity, and its interactions with environmental and lifestyle factors are likely just one part of a much larger picture. More research is needed to fully understand these complex interactions and their implications for pain management."
- Overall Health and Pain Sensitivity: Lifestyle factors like diet and exercise contribute to overall health and wellbeing, which can in turn impact pain sensitivity. For instance, regular exercise has been shown to improve pain tolerance and decrease pain perception. A balanced diet can reduce systemic inflammation, which can lower overall pain levels. These benefits might be particularly helpful for individuals with SCN9A variations that predispose them to higher pain sensitivity or specific pain disorders.
- Epigenetic Modifications:Lifestyle factors can influence epigenetic modifications, which are chemical changes to the DNA molecule or associated proteins that can affect gene expression without changing the underlying DNA sequence. These modifications can influence the expression of genes involved in pain perception, potentially including SCN9A. However, as of my last training data, the specifics of how lifestyle factors influence SCN9A expression through epigenetic changes remain unclear.
- Stress and Pain Sensitivity: Lifestyle factors that influence stress levels, including diet and exercise as well as other factors like sleep and mental health, can potentially interact with genetic factors to influence pain sensitivity. Stress can affect pain perception and may also interact with genetic predispositions to affect pain sensitivity.
Are there any known interactions between the SCN9A gene and psychological factors, such as stress or anxiety, in pain modulation?
Pain perception is a complex phenomenon that is influenced by a multitude of biological, psychological, and environmental factors. The SCN9A gene, which encodes the Nav1.7 sodium channel, plays a critical role in the transmission of pain signals in the peripheral nervous system. However, the direct interaction between SCN9A and psychological factors like stress or anxiety is less clear and requires more research. That being said, here are a few ways in which the SCN9A gene and psychological factors might interact to modulate pain:The precise interactions between SCN9A and psychological factors like stress or anxiety remain an active area of research. Understanding these interactions could have important implications for the management of pain conditions, potentially highlighting the need for integrated treatments that address both the biological and psychological aspects of pain."
- Pain Perception and Anxiety/Stress: High levels of anxiety or stress can amplify pain perception, a process known as pain catastrophizing. In individuals who have certain variations in the SCN9A gene that make them more sensitive to pain, high levels of anxiety or stress might exacerbate their pain experiences even further.
- Psychological Distress and Pain Thresholds: Psychological distress can lower the threshold at which a stimulus is perceived as painful. Variations in the SCN9A gene can also affect pain thresholds. Therefore, it's plausible that these factors could interact to modulate pain experiences.
- Neurobiological Links: Stress and anxiety can result in alterations to the nervous system that affect pain perception. For instance, these psychological states can lead to the release of stress hormones that sensitize the nervous system to pain. Given that SCN9A plays a key role in pain signaling in the nervous system, it's possible that such changes could interact with SCN9A function in ways that influence pain perception.
- Chronic Pain and Mental Health: Chronic pain conditions, such as those associated with certain SCN9A mutations, can lead to increased rates of depression, anxiety, and stress, which in turn can exacerbate the experience of pain.
What role does the SCN9A gene play in the development and maturation of the nervous system and pain circuits?
The SCN9A gene plays a critical role in the development and function of the nervous system, especially with respect to pain circuits. This gene provides instructions for making a protein that is critical for the proper function of nerve cells (neurons) and is specifically involved in the transmission of pain signals.The protein produced by SCN9A is a part of a family of proteins known as sodium channels, which are located in the membrane of neurons. These sodium channels are responsible for the initiation and transmission of electrical signals within neurons, which is crucial for normal communication between these cells.
In the context of pain, the sodium channel produced by SCN9A, known as Nav1.7, is predominantly found at the ends of pain-sensing nerves (nociceptors). The Nav1.7 channel plays a crucial role in the generation and conduction of pain signals. When there's a painful stimulus, Nav1.7 channels open, allowing sodium ions to flow into the cell, which generates an electrical signal that travels along the nerve to the spinal cord and ultimately the brain, where it's perceived as pain.
Mutations in SCN9A can lead to several conditions related to altered pain perception. For example, gain-of-function mutations (those that increase the activity of the Nav1.7 channel) can cause conditions characterized by episodes of severe pain, such as inherited erythromelalgia (IEM) and paroxysmal extreme pain disorder (PEPD). On the other hand, loss-of-function mutations (those that decrease or eliminate the activity of Nav1.7) can result in an inability to feel pain, as seen in a condition called congenital insensitivity to pain (CIP).
Therefore, SCN9A and its product Nav1.7 play a critical role in the development and maturation of pain circuits within the nervous system. Understanding the function and regulation of this gene and its associated protein can offer insight into new treatments for a range of pain-related conditions."
Can changes in the SCN9A gene expression or function contribute to the development of chronic pain conditions following acute injuries or surgeries?
"Yes, changes in the SCN9A gene expression or function could potentially contribute to the development of chronic pain conditions following acute injuries or surgeries. The Nav1.7 sodium channel, which is encoded by SCN9A, is a critical player in the transmission of pain signals. Changes in the functionality or expression of this channel can alter pain sensation and potentially contribute to the development of chronic pain conditions.Several pieces of evidence suggest a potential role of SCN9A in chronic pain:
Based on these findings, it's plausible that changes in SCN9A could also contribute to the development of chronic pain after acute injuries or surgeries. However, it's important to note that pain is a complex trait influenced by a multitude of factors, including other genes, environmental factors, and psychological factors. Therefore, while SCN9A could contribute to chronic pain conditions, it is just one piece of a very complex puzzle. Further research is needed to fully understand the role of SCN9A and other factors in the development of chronic pain conditions."
- Genetic variations in SCN9A have been found to be associated with a risk of developing certain chronic pain conditions. For example, some studies have found associations between specific SCN9A variants and the risk of developing conditions like chronic widespread pain and painful peripheral neuropathy.
- Animal studies have shown that the expression of Nav1.7 can be upregulated (increased) in response to nerve injury, which may contribute to heightened pain sensation.
- Research has shown that gain-of-function mutations in SCN9A, which enhance the activity of Nav1.7, can cause inherited erythromelalgia (IEM) and paroxysmal extreme pain disorder (PEPD), both of which are characterized by episodes of severe pain.
Are there any known natural or synthetic compounds that can modulate the activity of the SCN9A gene and potentially offer new avenues for pain management?
Yes, there are several compounds, both natural and synthetic, that are known to modulate the activity of the SCN9A gene, or more specifically, the Nav1.7 sodium channel that it encodes. These compounds can affect the function of Nav1.7 and, therefore, have potential as therapeutics for pain management. Some of these compounds include:These compounds represent potential starting points for the development of new pain treatments. However, developing drugs that selectively target Nav1.7 has proven to be challenging, and no Nav1.7-specific drugs have been approved for use in humans. Pain is a complex trait and managing it often requires a multi-faceted approach. Further research is needed to fully understand the role of Nav1.7 in pain and to develop effective and safe therapeutics based on this target."
- Tetrodotoxin (TTX): This is a potent blocker of sodium channels, including Nav1.7. TTX is derived from the pufferfish and certain other marine species. It's been investigated for its potential use in conditions such as cancer pain and chronic pain.
- Ziconotide (Prialt): This synthetic compound is derived from a peptide found in cone snail venom. It does not directly block Nav1.7, but it blocks other types of calcium channels that indirectly modulate the pain pathway. It is currently approved for severe chronic pain that cannot be managed with other treatments.
- A-803467, PF-05089771, and VX-150: These are synthetic small molecule inhibitors of Nav1.7 that have been studied in preclinical or early-stage clinical trials for their potential to treat pain.
- Spider and Scorpion toxins: Some toxins derived from spider and scorpion venoms have been found to specifically target Nav1.7 channels and modulate their activity. These toxins are being studied as potential pain therapeutics.
How does the SCN9A gene interact with other pain-related genes or genetic pathways to collectively influence pain perception?
Pain perception is a complex trait that's influenced by the interaction of many different genes and genetic pathways, and SCN9A is one of the key genes involved in this process. The protein that SCN9A encodes, Nav1.7, is an integral component of the pain signaling pathway in peripheral sensory neurons. SCN9A interacts with other pain-related genes and genetic pathways in several ways:The exact nature and extent of these interactions can be highly complex and depend on various factors, including the specific genetic context, environmental influences, and individual differences in pain perception. Unraveling these interactions is an active area of research and could provide valuable insights into the genetic basis of pain and the development of new pain treatments."
- Signal propagation: Nav1.7, produced by SCN9A, helps propagate electrical signals in sensory neurons. Other genes involved in signal propagation or neuron firing, such as those encoding other types of sodium channels (like SCN10A and SCN11A), potassium channels, and calcium channels, can therefore interact with SCN9A in terms of overall neuronal excitability and signal transmission.
- Signal modulation: Other genes encoding proteins that modulate Nav1.7 function can indirectly interact with SCN9A. For instance, genes encoding inflammatory molecules and growth factors, which can modulate the function and expression of Nav1.7 under conditions of injury or inflammation, may influence pain perception.
- Signal perception: The signals transmitted via Nav1.7 and other channels need to be interpreted by the brain to be perceived as pain. Therefore, SCN9A also interacts with genes involved in signal interpretation in the brain, like those involved in neurotransmitter release and binding.
- Genetic modifiers: Some genes may modify the pain phenotype associated with SCN9A mutations. For instance, a study published in 2016 suggested that certain variants in the COMT gene, which is involved in the breakdown of neurotransmitters, may modify the pain phenotype in people with specific SCN9A mutations.
Can the study of the SCN9A gene provide insights into the gender differences observed in pain sensitivity and pain disorders?
Yes, studying the SCN9A gene and its associated protein, Nav1.7, could provide insights into gender differences observed in pain sensitivity and pain disorders. Research has suggested that there are indeed gender differences in the experience of pain, with women generally reporting higher levels of pain and a greater prevalence of many chronic pain disorders.Several reasons could contribute to these differences, including hormonal influences, psychosocial factors, and possibly genetic factors. Some research has indicated that certain genes associated with pain perception, including SCN9A, may be differentially expressed or regulated in males and females. For example, sex hormones such as estrogen and testosterone could potentially influence the expression or function of Nav1.7, although the specifics of these interactions are not fully understood.
Moreover, there may be interactions between SCN9A and other genes on the X chromosome, which could potentially influence gender differences in pain perception. Given that females have two X chromosomes and males have one X and one Y chromosome, certain genetic variations might have differential effects in males and females. It's also worth noting that some studies have found associations between specific SCN9A variants and certain chronic pain conditions, and it's possible that the prevalence of these variants could differ between males and females.
However, while SCN9A is certainly an important gene for pain perception, it is just one piece of a very complex puzzle. Pain is influenced by a multitude of factors, including other genes, environmental factors, psychological factors, and more. Further research is needed to fully understand the complex interplay of these factors and their role in the gender differences observed in pain sensitivity and pain disorders."
What are the potential implications of studying the SCN9A gene for the development of personalised pain medicine and individualized treatment approaches?
The study of the SCN9A gene has significant potential for the development of personalized pain medicine and individualized treatment approaches. This gene encodes the Nav1.7 sodium channel, which plays a key role in the transmission of pain signals. Therefore, understanding variations in this gene could offer insights into why individuals experience pain differently and how they might respond to treatments. Here are a few implications of studying SCN9A for personalized pain medicine:While the potential is significant, it's important to remember that pain is a complex trait influenced by a multitude of factors, including other genes, environmental influences, and psychological factors. Therefore, while studying SCN9A is a crucial piece of the puzzle, a comprehensive understanding of these other factors is also needed for the development of truly personalized pain medicine."
- Identifying risk: Variations in SCN9A have been linked with different pain conditions. Understanding these genetic differences could help identify individuals who are at a higher risk of developing certain types of pain, potentially enabling early intervention or preventive strategies.
- Tailoring treatments: If certain genetic variants in SCN9A are found to be associated with the effectiveness of specific treatments, this could guide personalized treatment plans. For example, individuals with certain SCN9A variants might respond better to specific drugs that target the Nav1.7 channel.
- Developing new treatments: Research into SCN9A and the Nav1.7 channel could lead to the development of new treatments for pain. For instance, drugs that modulate the function of Nav1.7 could potentially offer relief for individuals who do not respond to current pain treatments.
- Understanding pain perception: Studying SCN9A could help us better understand why people perceive pain differently. This could ultimately lead to more effective pain management strategies tailored to an individual's unique pain perception profile.
How do genetic variations in the SCN9A gene contribute to the heterogeneity of pain phenotypes and treatment responses observed in clinical practice?
Pain is a complex trait that's influenced by a multitude of factors, including genetic, environmental, and psychological components. The SCN9A gene, which encodes the Nav1.7 sodium channel, plays a critical role in the transmission of pain signals, and genetic variations in this gene can significantly contribute to the heterogeneity of pain phenotypes and treatment responses observed in clinical practice.By studying genetic variations in SCN9A and their effects on pain perception and treatment response, clinicians and researchers can better understand the underlying biology of pain and develop more effective, personalized treatment strategies. However, it's important to remember that pain perception and response to treatment are complex traits that are influenced by a wide array of factors, and SCN9A is just one piece of this complex puzzle. Further research is needed to fully understand the role of genetics in pain and its treatment."
- Pain Phenotypes: Different mutations in the SCN9A gene can result in different pain disorders. For example, gain-of-function mutations, which increase the activity of the Nav1.7 channel, can cause conditions characterized by severe pain such as inherited erythromelalgia (IEM) and paroxysmal extreme pain disorder (PEPD). In contrast, loss-of-function mutations, which decrease or eliminate the activity of Nav1.7, can result in an inability to feel pain, a condition called congenital insensitivity to pain (CIP).
- Pain Threshold and Sensitivity: Even subtle changes or variations in the SCN9A gene can influence an individual's threshold and sensitivity to pain. Some studies suggest that certain genetic variations in SCN9A may be associated with differences in pain sensitivity in the general population.
- Treatment Responses: Variations in SCN9A could potentially influence responses to pain treatments. For instance, individuals with certain SCN9A variants might respond differently to specific pain medications. This could explain, in part, why some individuals do not respond to certain treatments or require higher or lower doses of medication.
- Chronic Pain Conditions: Research has indicated that certain variations in SCN9A may be associated with an increased risk of developing chronic pain conditions. This suggests that genetic factors could contribute to the transition from acute to chronic pain in some individuals.
Explain CRISPR-dCas9 epigenome editing methods to treat chronic pain by repressing Nav1.7 gene expression rather than via permanent genetic alteration.
https://newatlas.com/science/crispr-gene-therapy-opioid-free-chronic-pain-relief/
"The Nav1.7 gene is responsible for encoding a sodium channel involved in pain sensation in humans. Mutations in this gene can cause disorders related to pain sensitivity, including complete insensitivity to pain. So, it's an attractive target for pain management therapies.
Traditionally, CRISPR gene editing techniques have been used to make permanent changes to DNA by cutting the DNA sequence at specific points and either removing, repairing, or adding genetic material. However, these changes are irreversible and carry a risk of off-target effects or unanticipated consequences.
CRISPR-dCas9 is a modified form of the standard CRISPR gene editing system, where "dCas9" refers to "dead" Cas9. In contrast to the standard Cas9 enzyme, which cuts DNA at specific locations, dCas9 has been engineered to be catalytically inactive, i.e., it cannot cut DNA. Instead, it can be guided to specific genomic regions where it can modulate gene expression without making permanent changes to the DNA sequence.
In the context of treating chronic pain, CRISPR-dCas9 can be used for "epigenome editing." The epigenome refers to the set of chemical modifications to the DNA and DNA-associated proteins in a cell that control gene expression without changing the underlying sequence.
Here's how it would work for repressing Nav1.7 gene expression:
Outcome: With less Nav1.7 protein, the patient's sensitivity to pain would theoretically be decreased.
- Design: Scientists design guide RNAs (gRNAs) that match the sequence of the Nav1.7 gene. They also attach a repressor molecule to the dCas9 enzyme. The repressor molecule can inhibit gene expression when it's brought into proximity with a gene.
- Delivery: The dCas9, gRNAs, and repressor molecule are introduced into the patient's cells, often using a harmless virus as a vector.
- Binding and repression: Guided by the gRNA, the dCas9-repressor complex binds to the Nav1.7 gene's promoter region (the DNA region that initiates transcription of the gene). When the repressor molecule is in this location, it inhibits the machinery that would typically initiate gene expression, thereby reducing or stopping the production of the Nav1.7 protein.
This method's advantage is its reversibility. Unlike traditional CRISPR techniques, it doesn't permanently alter the DNA sequence. If the treatment causes unforeseen side effects or needs to be stopped for any reason, removing the dCas9 and gRNAs from the cell would allow gene expression to return to normal.
However, this type of therapy is still in experimental stages. More research is needed to fully understand its potential benefits, risks, and long-term effects."
Any reader who is the victim of chronic pain will probably find futuristic speculation on gene therapies maddeningly irrelevant. Pain-relief is needed now. What are currently the most effective pharmacotherapies for chronic pain?
"The management of chronic pain often requires a multi-faceted approach involving pharmacological and non-pharmacological methods. Here are some of the most commonly used types of pharmacotherapies for chronic pain:These treatments can be used alone or often in combination. The choice of treatment depends on the type and cause of pain, the patient's other health conditions, and their personal response to medication. A healthcare provider can provide guidance on the most suitable treatment options.
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): These are often the first line of treatment for many types of pain. Examples include ibuprofen (Advil) and naproxen (Aleve). They can be effective for musculoskeletal pain such as arthritis and are also used for headache and menstrual pain. However, long-term use can cause gastrointestinal issues and increase the risk of cardiovascular events.
- Acetaminophen (Paracetamol): Acetaminophen, also known as paracetamol or Tylenol, is used for mild to moderate pain. It has fewer side effects than NSAIDs but overuse can lead to liver damage.
- Antidepressants: Certain types of antidepressants, particularly tricyclic antidepressants (like amitriptyline) and serotonin and norepinephrine reuptake inhibitors (like duloxetine), can be effective for various types of chronic pain, including neuropathic pain and fibromyalgia.
- Anticonvulsants: Drugs originally developed to treat epilepsy, such as gabapentin and pregabalin, can be effective for neuropathic pain.
- Opioids: Opioids, such as morphine, oxycodone, and hydrocodone, are powerful pain relievers. However, they have significant side effects and risks, including the potential for addiction, and are usually reserved for severe pain that hasn't responded to other treatments. Their use needs to be closely monitored by a doctor.
- Topical treatments: Topical treatments such as capsaicin or lidocaine can be effective for localized pain. They are generally safe but can cause skin irritation in some people.
- Corticosteroids: These are powerful anti-inflammatory drugs that can be used for conditions like rheumatoid arthritis and lupus. They can be given orally or injected directly into the painful area.
- Muscle relaxants: Drugs such as cyclobenzaprine or baclofen can be used for conditions like muscle spasms or spasticity.
The development of new pain treatments is ongoing, and there may be more recent advances in the treatment of chronic pain. It's also worth mentioning that non-pharmacological treatments, such as physical therapy, psychological therapies (like cognitive-behavioral therapy), and lifestyle changes, are crucial parts of a comprehensive approach to managing chronic pain."
Does trivialising pain to "just a useful signalling mechanism" by gene therapy and/or germline editing (hedweb.com) risk diminishing empathy? Or do empathetic euphoriants like MDMA (mdma.net) demonstrate that radically enhanced well-being is consistent with enhanced compassion?
"Empathy and pain perception are complex traits with both biological and environmental influences. How alterations to pain perception might affect empathy is not straightforward and would likely depend on many factors.In summary, whether reducing pain perception might diminish empathy is a complex question without a simple answer. Empathy and pain perception are both multi-faceted traits, and their relationship is likely to be influenced by many factors beyond just the capacity to feel pain. It's conceivable that we could develop strategies to enhance both well-being and empathy, but this would require a nuanced understanding of the biological and environmental factors that shape these traits, as well as careful ethical consideration of the potential risks and benefits."
- Pain and empathy: Empathy often involves an ability to understand and share the feelings of others, which can be informed by our own experiences, including pain. Some might argue that if we reduce our ability to feel pain, we might struggle to empathize with the pain of others. However, empathy is not solely derived from personal experiences of pain. Empathy also depends on cognitive and affective processes, such as the ability to take the perspective of others, to understand their emotions, and to feel concern for their well-being.
- Empathetic euphoriants: Drugs like MDMA (ecstasy) are known to increase feelings of empathy and connectedness, while also enhancing mood. The effects of MDMA involve multiple neurotransmitter systems, including serotonin, dopamine, and norepinephrine, and it also has effects on hormones like oxytocin, which is involved in social bonding. These effects demonstrate that it's possible, in principle, to have states of enhanced well-being that are also characterized by increased empathy and compassion. However, while these substances can temporarily alter empathy and mood, they do not represent a long-term or sustainable strategy for enhancing well-being or empathy.
- Gene therapy and germline editing: Using gene therapy or germline editing to modify pain perception could have various effects on empathy, depending on what specific changes are made and how they interact with a person's other characteristics and their environment. If such modifications led to a general increase in well-being and a reduction in personal suffering, one could hypothesize that this might free up cognitive and emotional resources that could then be directed towards understanding and helping others, potentially increasing empathy.
List genetic conditions other than nonsense mutations of the SCN9A gene that induce congenital insensitivity to pain.
"Congenital insensitivity to pain (CIP) is a rare condition, and while nonsense mutations in the SCN9A gene are a common cause, there are other genes and genetic conditions implicated as well. Here are some of them:Remember, these conditions are incredibly rare and might not solely present with insensitivity to pain. They may also include other neurological or physical symptoms."
- PRDM12 gene mutations: This gene is involved in the development of pain-sensing nerve cells. Mutations can lead to congenital insensitivity to pain.
- NTRK1 gene mutations (Congenital insensitivity to pain with anhidrosis): This gene encodes a receptor for nerve growth factor, which is crucial for the survival and differentiation of neurons. Mutations cause a syndrome that includes insensitivity to pain.
- FAM134B gene mutations (Hereditary sensory and autonomic neuropathy type II): This gene is involved in the long-term survival of nerve cells. Mutations cause a syndrome that includes a reduction in the ability to feel pain and temperature.
- HSN2 gene mutations (Hereditary sensory and autonomic neuropathy type II): Mutations in this gene cause a form of HSAN II, a condition that includes reduced ability to feel pain.
- WNK1 gene mutations (Hereditary sensory and autonomic neuropathy type II): Mutations in this gene can also cause HSAN II, leading to the inability to feel pain.
- NGFB gene mutations (Hereditary sensory and autonomic neuropathy type V): This gene encodes nerve growth factor beta, and mutations cause an inability to feel pain, pressure, and heat.
- SPTLC1 and SPTLC2 gene mutations (Hereditary sensory and autonomic neuropathy type I): These genes are involved in the synthesis of certain types of fats that are found in cell membranes, including nerve cells. Mutations can lead to a reduced ability to feel pain.
Do some people with congenital insensitivity to pain lead normal lives?
"Congenital insensitivity to pain (CIP) is a rare condition that affects the nervous system and results in the inability to perceive physical pain. This might sound like an advantage at first, but it can actually be quite dangerous. Pain serves as a warning signal to the body to avoid harm, and without this signal, people with CIP can easily injure themselves without realizing it.For example, a child with CIP may not notice that they've broken a bone or been burned. These unnoticed injuries can lead to severe complications, like infections, joint damage due to untreated fractures, or complications from burns. People with CIP may also bite their tongue, cheeks, or fingers to the point of mutilation, especially when they are younger and don't understand the consequences of their actions.
Despite these challenges, some people with CIP can lead relatively normal lives, particularly if the condition is diagnosed early and carefully managed. They require continuous care and monitoring for unnoticed injuries and have to be taught to visually monitor for potential harm, like swelling or redness, that might suggest an injury. They also need to avoid situations that could potentially cause harm and should receive regular medical checkups to catch and treat any unnoticed injuries or conditions.
The severity of CIP and the extent to which it affects an individual's life can also vary depending on the specific genetic mutation causing the condition. Some individuals may have certain residual sensations that can aid in recognizing harmful situations.
But in general, having congenital insensitivity to pain requires significant lifestyle modifications and ongoing medical care to prevent and manage injuries and complications. It's important to remember that what's considered "normal" can vary widely from person to person and that quality of life is a deeply personal measure."
Describe other genes besides SCN9A involved in nociception and pain processing.
"Pain perception is a complex process that involves the coordinated action of multiple genes and pathways. While SCN9A is one of the most well-studied genes in pain perception due to its role in encoding the Nav1.7 voltage-gated sodium channel, it's not the only gene involved in this process. Other genes that have been found to play significant roles in pain perception include:Remember, the genetics of pain perception is a complex field, and these genes represent just a small portion of the many genes likely involved in this process. In addition, many of these genes may interact with each other and with environmental factors to influence pain perception in ways that are not fully understood."
- TRPV1: This gene encodes the TRPV1 protein, a heat-sensitive member of the transient receptor potential (TRP) family of ion channels. This protein is primarily expressed in nociceptive neurons and is activated by capsaicin (the compound responsible for the 'burn' of hot peppers), heat, and low pH (acidic conditions). Mutations in this gene have been associated with altered pain sensitivity.
- COMT (Catechol-O-methyltransferase): The COMT gene is involved in the metabolic breakdown of certain neurotransmitters, including dopamine, epinephrine, and norepinephrine. Variations in this gene have been associated with differences in pain perception and susceptibility to chronic pain conditions.
- OPRM1: This gene encodes the mu opioid receptor, the primary target of opioid analgesics like morphine. Genetic variations in this gene can affect individuals' responses to opioid medications and may be linked to differences in pain sensitivity and risk of developing chronic pain.
- TRPA1: Another member of the transient receptor potential (TRP) family of ion channels, the TRPA1 channel is activated by cold temperatures and a variety of chemical irritants. Mutations in this gene have been implicated in familial episodic pain syndrome, a rare disorder characterized by episodes of severe pain in the upper body.
- SCN10A: This gene encodes the Nav1.8 voltage-gated sodium channel, which is also predominantly expressed in nociceptive neurons. Like SCN9A, mutations in SCN10A have been linked to altered pain sensitivity.
- FAAH (Fatty Acid Amide Hydrolase): The FAAH gene is involved in the breakdown of endocannabinoids, natural compounds in the body that play a role in pain modulation among other things. Variations in this gene can affect individuals' pain sensitivity and responses to certain analgesic drugs.
Describe the role of different COMT (Catechol-O-methyltransferase) alleles in default levels of physical and emotional well-being / ill-being.
"Catechol-O-methyltransferase (COMT) is an enzyme involved in the metabolism and breakdown of catecholamines, which include neurotransmitters such as dopamine, epinephrine (also known as adrenaline), and norepinephrine. These neurotransmitters play a critical role in functions such as mood regulation, cognitive function, stress response, and pain perception.There is one particularly well-studied single-nucleotide polymorphism (SNP) in the COMT gene, known as Val158Met (rs4680), which results in a substitution of the amino acid valine (Val) by methionine (Met) at position 158.
It's important to note that the relationship between COMT variants and psychological traits or predispositions is complex, and likely influenced by many other genetic and environmental factors. Additionally, the scientific understanding of these relationships is evolving and some findings are controversial, with different studies sometimes producing conflicting results. More research is needed to fully understand the implications of different COMT genotypes on physical and emotional well-being."
- Val/Val variant: Individuals with the Val/Val genotype have a version of COMT that is more thermally stable and has up to four times the enzymatic activity of the Met variant. This leads to more rapid degradation of dopamine and may be associated with lower levels of this neurotransmitter in the prefrontal cortex, a region of the brain involved in cognitive function and emotional regulation. Some research suggests that individuals with the Val/Val genotype may have higher pain thresholds and lower risk of developing chronic pain. However, they may also be at higher risk for stress-related psychopathologies, as faster dopamine degradation could result in poorer emotional regulation under stress.
- Met/Met variant: The Met/Met variant, on the other hand, is associated with slower dopamine degradation, leading to higher levels of this neurotransmitter in the prefrontal cortex. This may provide cognitive advantages, such as better performance on tasks requiring executive function, and potentially better emotional regulation under stress. However, this variant has been associated with lower pain thresholds and a higher risk for chronic pain conditions.
- Val/Met variant: Individuals with the heterozygous Val/Met genotype typically display intermediate COMT activity and traits between the two homozygous states.
Describe the role of different FAAH and FAAH-OUT alleles in default levels of physical and emotional well-being / ill-being.
https://www.theguardian.com/science/2019/mar/28/scientists-find-genetic-mutation-that-makes-woman-feel-no-pain
"Fatty acid amide hydrolase (FAAH) is an enzyme that breaks down endocannabinoids in the body. Endocannabinoids are naturally occurring compounds that bind to the same receptors as THC, the primary psychoactive compound in cannabis. One of the most well-studied endocannabinoids is anandamide, a compound that plays a role in pain regulation, mood, appetite, and other functions.
A specific variant in the FAAH gene has been linked to increased pain tolerance and decreased anxiety and fear. The variant, known as C385A or rs324420, leads to a substitution of the amino acid proline by threonine, resulting in reduced FAAH activity and increased anandamide levels. Studies suggest that individuals with this variant may be less anxious, less prone to stress, have a higher pain threshold, and possibly have a decreased risk for certain psychiatric disorders. However, they may also have an increased risk for certain problematic behaviors, such as drug use and problem gambling.
FAAH-OUT is a so-called "pseudogene" adjacent to FAAH. It was traditionally thought to be non-functional, but recent research has shown that it may play a role in regulating the expression of the FAAH gene. A mutation that deletes a section of the FAAH-OUT gene was identified in a woman who has an extremely high pain threshold and never experiences anxiety or fear. The mutation is believed to increase the expression of the FAAH gene, resulting in elevated levels of anandamide and the observed effects on pain, anxiety, and fear.
It's important to note that while these findings are promising, the relationships between these genes, endocannabinoid levels, and physical and emotional well-being are likely complex and influenced by other factors. Mre research is needed to fully understand these relationships and their implications for human health and well-being."
Describe ways to design ideal alleles of SCN9A, COMPT, FAAH and FAAH-OUT genes to maximize default lifetime well-being of future transhuman, human and nonhuman animals as advocated in The Hedonistic Imperative.
"The Hedonistic Imperative, as proposed by philosopher David Pearce, advocates for the use of biotechnology to genetically engineer happiness, phasing out suffering in all sentient life. This includes modifying the genetic code of future generations of humans and non-human animals to maximize their well-being.Designing ideal alleles of the SCN9A, COMT, FAAH, and FAAH-OUT genes is a challenging task due to the complexity of genetic interactions and the multifaceted nature of well-being. However, here's a general idea of how each gene might be targeted:
However, it's essential to remember that these are simplistic descriptions. Genes don't operate in isolation, and small changes can have broad and unpredictable effects due to the complexity of genetic networks. Plus, what's "ideal" could vary greatly between individuals due to genetic background, environmental influences, and personal circumstances.
- SCN9A: This gene codes for a voltage-gated sodium channel (Nav1.7) involved in pain sensation. An ideal allele would likely reduce the function of this channel to decrease the intensity of pain signals. However, it's important to maintain some functionality to allow for the detection of harmful stimuli (nociception). Variants like the rs6746030 (R1150W) could serve as a basis for design.
- COMT: The COMT gene is involved in the metabolism of neurotransmitters like dopamine. An ideal allele could potentially increase the stability or efficiency of the COMT enzyme, resulting in optimal dopamine levels that enhance mood and cognition without leading to dysregulation or disorders like ADHD or schizophrenia.
- FAAH: This gene codes for an enzyme that breaks down anandamide, a molecule that plays a crucial role in mood, appetite, and pain. A desirable FAAH allele would balance the activity of the FAAH enzyme to maintain optimal anandamide levels, maximizing well-being and reducing pain without causing issues like obesity or dependency.
- FAAH-OUT: As a pseudogene, FAAH-OUT might regulate the expression of FAAH. Ideal alterations could involve ensuring its regulatory role enhances anandamide levels in a balanced way, contributing to optimal mood and pain regulation.
Finally, from an ethical standpoint, any form of genetic engineering should prioritize the autonomy, consent, and welfare of those affected. The implications are especially complex when considering transhuman or non-human applications, as issues of consent and understanding become even more challenging. Moreover, the safety and potential unforeseen consequences of genetic manipulation are significant considerations, necessitating stringent scientific and ethical scrutiny before such interventions are considered."
All sexual reproduction involves untested genetic experiments. All procreation involves bringing new life and suffering into the world without the prior consent of the victim. Make the case for why responsible parenthood involves trying to minimise the potential lifetime mental and physical pain of one's future children.
"The case for minimizing potential lifetime mental and physical pain in prospective children aligns with a deep and universal principle: the ethical duty of care that prospective parents have towards their future offspring.The concept of reducing potential suffering through genetic means is an extension of what responsible parents already do: aim to provide their children with the best possible life. If we acknowledge that genetic factors significantly influence one's life trajectory, it's a logical extension to use our knowledge and tools to make that trajectory as positive as possible."
- Ethical Duty of Care: Parents generally feel a responsibility to protect their children from harm and suffering. This principle extends not just to their children's upbringing but also to their creation. If we have the capacity to minimize future suffering, we could argue that we have a moral obligation to do so.
- Avoiding Unnecessary Suffering: If we have the means to identify and potentially reduce genetic predispositions to pain, mental health disorders, or physical diseases, it could be viewed as irresponsible not to take these precautions.
- Informed Decisions: With the advancement of genetic screening and gene-editing technologies, parents are increasingly able to make informed decisions about the potential health outcomes of their future children. This could include decisions aimed at minimizing potential suffering.
- Promoting Well-Being: By reducing the potential for physical and mental suffering, we can increase the chance that future individuals will lead lives characterized by well-being and flourishing.
- Legacy of Care: The genetic decisions we make today have implications not only for our immediate offspring but also for countless future generations. By thoughtfully considering these decisions, we can contribute to a legacy of reduced suffering and increased well-being.
Sexual reproduction is currently a genetic crapshoot. Describe the protocol for a large, well-controlled trial using human prospective parents of pre-selected benign alleles of the SCN9A, COMPT, FAAH and FAAH-OUT genes to maximize default lifetime well-being of their future children.
"Designing a large, well-controlled trial to study the effects of pre-selected benign alleles of the SCN9A, COMT, FAAH, and FAAH-OUT genes in prospective human parents would involve many practical and ethical challenges. Here's a simplified blueprint of how such a protocol might look like, assuming all necessary ethical and legal approvals have been obtained:This is a simplified protocol, and in reality, the process would be much more complex and fraught with ethical and practical challenges. These include but are not limited to: ethical concerns about the use of gene editing in human embryos, potential off-target effects of gene-editing techniques, the inability to fully predict or control the outcomes of genetic modification, and the potential long-term effects on the child's health and well-being. It's important to remember that any intervention of this kind should be undertaken with extreme caution and only after thorough ethical, legal, and scientific scrutiny."
- Selection of Participants:
Identify a large cohort of prospective parents willing to participate in the trial. These should be healthy individuals who are planning to conceive a child. Extensive genetic counseling should be provided to explain the aims, benefits, potential risks and uncertainties of the trial.- Preimplantation Genetic Screening and Editing:
Collect eggs and sperm from the prospective parents for in-vitro fertilization (IVF). Once fertilized, use genetic screening techniques to analyze the genotypes of the embryos for the SCN9A, COMT, FAAH, and FAAH-OUT genes. For embryos without the pre-selected benign alleles, apply gene-editing techniques like CRISPR to introduce these alleles.- Implantation and Follow-up:
Implant the edited embryos in the mother and monitor the development and health of the fetus. After birth, monitor the child's health, well-being, pain tolerance, emotional health, cognitive abilities, and other relevant metrics regularly over their lifetime.- Data Collection and Analysis:
Collect and analyze data on the child's health and well-being, comparing the children with the pre-selected alleles to a control group of children conceived naturally or via IVF without gene editing.- Confidentiality and Consent:
Maintain strict confidentiality of participant data and ensure ongoing informed consent from parents (and eventually the child when they become of age) at every stage of the trial.Today's human and nonhuman animals can't benefit from benign alleles of the SCN9A, FAAH, FAAH-OUT and COMT genes. Outline how gene therapy could be used to benefit existing sentience.
https://www.nature.com/articles/d41586-021-01776-4
"Gene therapy offers promising prospects for modifying the genetic code of existing individuals to promote health and well-being. The primary goal is to introduce, remove, or alter genetic material within a person's cells to prevent or treat disease. While gene therapy is still a relatively new and experimental field, its potential applications continue to expand.
"The approach to gene therapy would vary depending on the specific gene in question. Here are some general steps for applying gene therapy to the SCN9A, FAAH, FAAH-OUT, and COMT genes:Applications of these steps to the four genes could include:
- Identify the Target: The first step in gene therapy is to determine which gene or genes need to be targeted for therapy. For SCN9A, FAAH, FAAH-OUT, and COMT genes, variants associated with increased well-being and reduced pain could serve as the therapeutic goal.
- Vector Development: Gene therapy requires a delivery mechanism or vector. Viruses are the most common type of vector used in gene therapy. They are modified to be safe and carry the therapeutic genes into cells. Development of an effective vector that can specifically target relevant cells (e.g., neurons for pain perception) is a significant part of this process.
- Gene Modification: The vector is used to carry and introduce the new, modified gene into the patient's cells. This can be done either in vivo (directly into the patient's body) or ex vivo (cells are modified outside the body and then reintroduced).
- Monitoring: After therapy, patients would be closely monitored to assess the effects of the treatment, manage potential side effects, and ensure the modified gene is functioning as intended.
However, it's essential to remember that gene therapy is currently primarily used for life-threatening genetic disorders, with many procedures still in experimental stages. The process involves potential risks, such as immune reactions, off-target effects, and the possibility of inducing cancer. Ethical considerations, including informed consent and the potential for unforeseen consequences, must be rigorously examined before any new gene therapy procedure.
- SCN9A: Gene therapy could potentially modify the SCN9A gene to reduce the intensity of pain signals, similar to people naturally carrying the 'low pain' rs6746030 variant.
- COMT: Therapy could aim to modify the COMT gene to optimize the function of the COMT enzyme, potentially promoting emotional well-being by regulating dopamine levels.
- FAAH and FAAH-OUT: Gene therapy could be used to balance the breakdown of anandamide, a molecule that plays a critical role in mood, appetite, and pain sensation.
Moreover, our understanding of the exact roles and interactions of the SCN9A, FAAH, FAAH-OUT, and COMT genes, particularly in relation to well-being, is still evolving. Therefore, while the prospect of using gene therapy to increase well-being and reduce suffering is exciting, it's a goal that requires much more research and understanding."
Many potent Nav1.7 blockers are only relatively weak analgesics, presumably because congenital loss of Navv1.7 caused by of nonsense mutations of the SC9A gene results in a dramatic increase in the levels of endogenous enkephalins. Does unusually high enkephalin function induce unusually good mood and depression-resistance? Or is this link a conjecture that warrants further research?
"Enkephalins are one of the types of endogenous opioids produced by the body. They primarily bind to the delta opioid receptors, but also have some affinity for the mu opioid receptors. They are involved in a wide range of physiological processes, including pain modulation and emotional regulation.In the context of Nav1.7 blockers, as you mentioned, it's believed that the increase in endogenous enkephalins caused by the loss of Nav1.7 function contributes significantly to the insensitivity to pain seen in individuals with nonfunctional Nav1.7 channels. This compensatory increase in enkephalins helps to further reduce the transmission of pain signals.
As for the role of enkephalins in mood regulation and resistance to depression, there's indeed evidence suggesting that the endogenous opioid system, including enkephalins, is involved in these processes. For instance, opioid receptors have been implicated in the regulation of mood, reward, and stress responses.
However, the precise relationship between enkephalin levels and mood is complex and likely depends on a variety of factors, including the specific areas of the brain where these opioids are acting, the balance of activity at different opioid receptors, and interactions with other neurotransmitter systems.
The link between unusually high enkephalin function and unusually good mood or depression-resistance is still not fully established and would indeed be a topic for further research. It's possible that such a relationship could exist, but establishing this would require carefully designed studies to control for potential confounding factors and to determine the direction of causality."
How can CRISPR-based synthetic gene drives be used to spread benign "low pain" alleles of SCN9A across the biosphere even if the benign variants in question would normally carry a modest fitness cost to the organism (gene-drives.com)
"Gene drives are a powerful technology that can potentially be used to spread specific genetic traits through a population more rapidly than would occur through natural selection. They work by biasing the inheritance of certain genes, so that these genes are passed on to offspring at a rate higher than the typical 50% chance.CRISPR, a gene-editing tool, can be used to create synthetic gene drives. By creating a gene drive with CRISPR, a specific allele (variant) of a gene can be made to "drive" itself into future generations, regardless of whether or not it provides a survival advantage. In other words, the allele is 'selfish' and gets passed on more often than you would expect based on standard Mendelian inheritance.
In the context of the SCN9A gene and the Hedonistic Imperative, theoretically, one could use a CRISPR-based gene drive to spread "low pain" alleles of SCN9A throughout a population, even if these alleles normally carry a modest fitness cost.
Here is a simplified step-by-step description of how this might work:
It's important to note, however, that the use of gene drives, especially in humans, raises numerous ethical, safety, and regulatory issues. It's not something that would be undertaken lightly or without thorough consideration and controls. The technology is largely theoretical and experimental, and has not been used in humans.
- Scientists first identify the "low pain" allele of SCN9A they wish to spread. They also identify a 'target' sequence in the SCN9A gene where they want to insert the "low pain" allele.
- Using CRISPR technology, scientists design a genetic construct that includes: the "low pain" allele of SCN9A, the CRISPR enzyme (usually Cas9), and guide RNAs that target the insertion site in the SCN9A gene.
- This genetic construct is then inserted into the germline cells (sperm or egg cells) of an organism.
- When these germline cells form an organism, and that organism mates, the gene drive goes into action. In the offspring, the CRISPR system cuts the SCN9A gene at the target location on the chromosome that did not inherit the gene drive. This damage triggers the cell's repair mechanisms.
- Instead of just fixing the cut, however, the cell uses the "low pain" allele from the gene drive as a repair template. This process, known as homology-directed repair, effectively copies the gene drive from one chromosome to the other.
- As a result, instead of only half of the offspring inheriting the gene drive (as would be the case with normal inheritance), nearly all of them do. These offspring can then pass the gene drive on to subsequent generations.
The concept of using gene drives to propagate "low pain" alleles across the biosphere could also have unforeseen ecological consequences, since organisms across the biosphere play key roles in their ecosystems, and changes to these organisms could have cascading effects. Pain, while often seen as negative, plays a crucial role in survival for many species.
Finally, the use of gene drives could potentially face technical issues such as the development of resistance in target organisms. Much more research is needed to fully understand and address these issues."
Some transhumanists (hedweb.com) believe we should aim for a more civilized signaling system beyond the age-old pleasure-pain axis, namely life based entirely on information-sensitive gradients of bliss: a pleasure-superpleasure axis. Compare the cerebral analogue to lovemaking, which has peaks and troughs but if done properly is generically pleasurable throughout. How do today's high-functioning genetic outliers like anandamide-rich Jo Cameron or Anders Sandberg ("I do have a ridiculously high hedonic set-point") hint that such a hyperthymic civilisation is technically feasible?
"The theory proposed by some transhumanists envisions a future where life is built on a continuum of pleasure, where even the lower ends of the spectrum are still enjoyable experiences, essentially replacing the current pleasure-pain axis with a pleasure-superpleasure axis.As you mentioned, a cerebral analogue to this concept can be likened to lovemaking, where the entire experience is pleasurable, albeit with varying intensities. This suggests that our nervous systems are already capable of encoding and experiencing such gradients of positive affect, which could be the basis for this new paradigm.
Examples of high-functioning genetic outliers like Jo Cameron and Anders Sandberg serve as living proof of the incredible diversity and potential flexibility of human experience:
These examples suggest that a "hyperthymic" civilization, characterized by a persistently positive mood state, could be technically feasible. It's conceivable that with advanced genetic engineering techniques, we could identify the key genetic factors contributing to an elevated hedonic set-point and manipulate them to achieve a state of existence based on gradients of bliss.
- Jo Cameron: Jo has a rare genetic mutation in the FAAH gene and the previously unknown FAAH-OUT gene. As a result, she produces higher than average levels of anandamide (the "bliss molecule") which makes her virtually immune to anxiety and fear and she can't experience physical pain. Her case suggests that it's possible for a human to have an elevated hedonic set-point due to genetic factors, and it hints at the potential for us to genetically modify these systems to increase overall well-being.
- Anders Sandberg: Anders, a well-known transhumanist, reports he has a "ridiculously high hedonic set-point". While the genetic basis for this isn't as clearly established as Jo's, his case does suggest that there are likely other genetic, environmental, and psychological factors that could be manipulated to increase one's baseline level of happiness.
However, such an undertaking would come with significant ethical and practical challenges. For instance, it would require a profound understanding of the complex genetics of mood and cognition, and it would need to ensure these changes are beneficial overall and do not lead to unintended side effects. Moreover, the idea of genetically modifying humans to be persistently happy raises complex questions about the nature of happiness and the human experience."
* * *
HOME
ChatGPT Overview
ChatGPT on Abolitionism
ChatGPT on Antinatalism
ChatGPT on Utilitarianism
ChatGPT on Antispeciesism
ChatGPT on Transhumanism
ChatGPT on Brave New World
The Wit and Wisdom of ChatGPT
ChatGPT on Utopian Pharmacology
ChatGPT on The Hedonistic Imperative
ChatGPT on Non-Materialist Physicalism
ChatGPT on The Reproductive Revolution
ChatGPT on The Biointelligence Explosion